Abstract
The endothelial cell adhesion molecule, CD146, is expressed on ≍ 2% of normal circulating T cells, correlating with T cell activation, endothelial interactions and T helper type 17 (Th17) effector functions. In this study, we have characterized CD146 expression in circulating T cells from healthy controls and patients with stable, well-controlled autoimmune connective tissue diseases (CTDs). In vitro, anti-CD3/anti-CD28 stimulation induced CD146 expression in both CD4 and CD8 T cells. In healthy controls and CTD patients, CD146 was associated with expression of recent and chronic activation markers (CD25+, OX-40+, CD69+, CD27–) and was confined to CD45RO+/RA–/CD28+ populations within the CD4 subset. Except for CD69, these markers were not associated with CD146 in the CD8 subset. Surprisingly, most CTD patients exhibited no T cell hyperactivation ex vivo. In five of five patients with secondary Sjögren's syndrome circulating T cells appeared activated despite therapy, and CD146 up-regulation, associated with activation markers, was observed both on CD4 and CD8 T cells. There was no association between CD146 and putative pro-atherogenic T cell subsets. In conclusion, the relationship of CD146 expression to T cell activation differs between T cell subsets in healthy subjects and correlates with systemic hyperactivity, where present, in patients with CTDs, as exemplified by the patients with secondary Sjögren's syndrome in this study.
Keywords: autoimmune connective tissue diseases, CD146/MelCAM, flow cytometry, T lymphocyte subsets, vascular/immune interactions
Introduction
CD146/melanoma cell adhesion molecule (MelCAM) is an immunoglobulin superfamily glycoprotein expressed at endothelial tight junctions on vascular smooth muscle cells and trophoblast cells, and variably on malignant melanoma cells [1,2]. Human T cells induce CD146 expression after mitogen stimulation [3]. In vivo, CD146+ T cells are enriched in delayed-type hypersensitivity lesions [3]; cerebrospinal fluid in multiple sclerosis [4]; and synovial effusions, tissue and blood in inflammatory arthritis [3,5] (C. Wu, R. Busch, J.S.H. Gaston, unpublished). CD146 is present on 1–2% of circulating T, B and natural killer (NK) cells of healthy humans [6,7], whereas murine CD146 is expressed on neutrophils and NK cells [8]. In these studies, CD146 on CD4+ cells was associated with activation and memory markers, increased adhesion to cytokine-activated endothelia and T helper type 17 (Th17) (and Th1) effector functions. Transfection of CD146 into lymphoid cell lines reorganizes adhesion molecules on microvilli and facilitates rolling interactions with endothelia [9].
In this study, we examined CD146 expression on circulating T cells from patients with autoimmune connective tissue diseases (CTDs), which were reported previously to exhibit phenotypic activation, effector cytokine production and derangement of memory/effector subsets ex vivo (reviewed in [10,11]). Patients with CTDs, particularly lupus, are at increased risk for atherosclerosis. This is not explained fully by conventional risk factors or side effects of therapy, due probably to exacerbation of the inflammatory component of atherosclerosis by autoimmunity [12–14]. Different CTDs exhibit different patterns of vascular involvement [15–17]. The immune component of atherosclerosis involves infiltration of atherosclerotic plaques by CD4+CD28− (late effector/senescent) T cells, expressing CCR5 and Th1 cytokines [18]. Therefore, we also tested whether CD146 expression correlates with pro-atherogenic T cell phenotypes.
Materials and methods
Human subjects
Patients with systemic lupus erythematosus (SLE), systemic sclerosis (SSc) or primary or secondary Sjögren's syndrome (pSS or sSS) were recruited through the CTD Clinic and the Vasculitis Clinic at Addenbrooke's Hospital, Cambridge, UK. Healthy donors (HDs) were recruited through the Department of Clinical Pharmacology. SLE patients fulfilled at least four ACR criteria, as revised in 1982 [19] and 1997 [20]. SSc patients met a recently revised set of criteria [21], and pSS patients followed the criteria of the European Union/United States consensus [22]. Patients with sSS met criteria for Sjögren's syndrome plus another CTD (SLE or SSc). The clinical characteristics of all patients are summarized in the online Supporting information, Table S1. Healthy individuals were screened to exclude those with autoimmune/inflammatory disease, and their history of cardiovascular disease was obtained. Pregnant women and smokers were excluded. Ethical approval was obtained (Norfolk REC 07/H0310/178), and all volunteers gave informed consent.
Flow cytometry
Peripheral blood was collected in 9-ml heparinized tubes and subjected to Ficoll density gradient centrifugation. Peripheral blood mononuclear cells (PBMCs) were isolated from the gradient interface and cryopreserved in 10% dimethylsulphoxide (DMSO)/90% heat-inactivated fetal bovine serum (FBS). Thawed PBMCs were washed and suspended in fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS)/1% bovine serum albumin/0·05% sodium azide] at 4 × 106 cells/ml. Aliquots (50 μl) were incubated in a 96 U-well plate with cocktails of fluorochrome-conjugated monoclonal antibodies (mAbs) in the dark for 45 min at 4°C, washed, suspended in FACS buffer and transferred into 12 × 75 mm tubes (Falcon, BD Ltd, Pontypridd, UK). The following antibodies (and corresponding isotype controls) were used, after titration (all from BD Biosciences, San Jose, CA, USA): CD25, CD69, CD70, human leucocyte antigen D-related (HLA-DQ), HLA-DR, OX40, CD40L, CD45RO, CD27, CD28 or CD31 [fluorescein isothiocyanate (FITC)]; CD45RA, CD54, or CXCR3 [antigen-presenting cells (APC)]. CD4-peridinin chlorophyll protein (PerCP) and CD146-phycoerythrin (PE) were included in all analyses. Some cocktails contained CD3-Alexa488 along with an APC-conjugated subset marker; others contained CD3-APC along with a FITC-conjugated subset marker. Intracellular staining with forkhead box protein 3 (FoxP3)-APC (eBioscience, San Diego, CA, USA) was performed as per the manufacturer's instructions, following surface staining for CD3, CD4 and CD146, using 5 × 105 cells per well. Some marker combinations were studied in only a subset of patients.
Analysis was performed using a FACSCantoII flow cytometer running FACSDiva software (BD Biosciences). In order to estimate low expression frequencies, 50 000–100 000 events were recorded per sample. Singlet lymphocytes were gated based on forward-scatter peak height versus peak area. Dead cells with reduced forward-scatter were excluded (as much as possible without use of viability dyes), but lymphocytes with larger forward-scatter, including activated cells undergoing blast transformation, were included. CD8 T cells were identified as CD3+CD4− cells; this approach yielded similar frequencies of CD146+ cells as positive staining for CD3 and CD8 (Supporting information, Fig. S1). Moreover, cryopreservation did not alter substantially the frequency of T cells expressing CD146 (Supporting information, Fig. S2).
In-vitro activation
Fresh PBMC from healthy donors were cultured in complete RPMI-1640 [Gibco, Carlsbad, CA, USA; with 5% human AB+ serum, 10 mM HEPES, non-essential amino acids, sodium pyruvate, 2 mM L-glutamine (Sigma, St Louis, MO, USA), 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA)] at 0·5 × 106 cells per 100 μl medium per well. T cells were stimulated with plate-bound anti-CD3 (HIT3a, coated onto microwells at 0·01, 0·1 or 1 μg/ml in PBS overnight) and soluble anti-CD28 (BD Biosciences; 0·1 μg/ml). PBMCs were cultured in a humidified incubator at 37°C with 5% CO2 for up to 4 days and analysed by flow cytometry.
Statistics
Percentages of CD4+ and CD4− T cells expressing CD146 and/or other markers were determined. Statistical analysis was performed using GraphPad Prism (version 4.02). Differences in subset frequencies between patient populations were compared by analysis of variance (anova) on ranks (Kruskal–Wallis test) with Dunn's multiple comparison. The Wilcoxon signed-rank test was used to compare the frequencies of two T cell subpopulations within each donor. P-values of less than 0·05 were reported as significant.
Results
Clinical and demographic characteristics of study populations
Peripheral blood was obtained from healthy, non-smoking donors (HD; n = 24), who were predominantly female (F : M = 15:9; none of the phenotypes investigated showed significant sex bias). Their median age was 61·5 years [interquartile range (IQR) = 34–68; range, 21–77]. Five HDs had a cardiovascular history (myocardial infarction, n = 2; hypertension, n = 2; angina, n = 1).
Patients with SLE (n = 14), SSc (n = 5), pSS (n = 7) and sSS (n = 5) were recruited. These patients were, with few exceptions (one SLE and one SSc), female; median ages ranged from 48 to 63 years in different groups. Clinically, patients had mild to moderate disease activity and were stable on current therapy (Supporting information, Table S1).
Activation-induced CD146 expression on CD4 and CD8 T cells in vitro
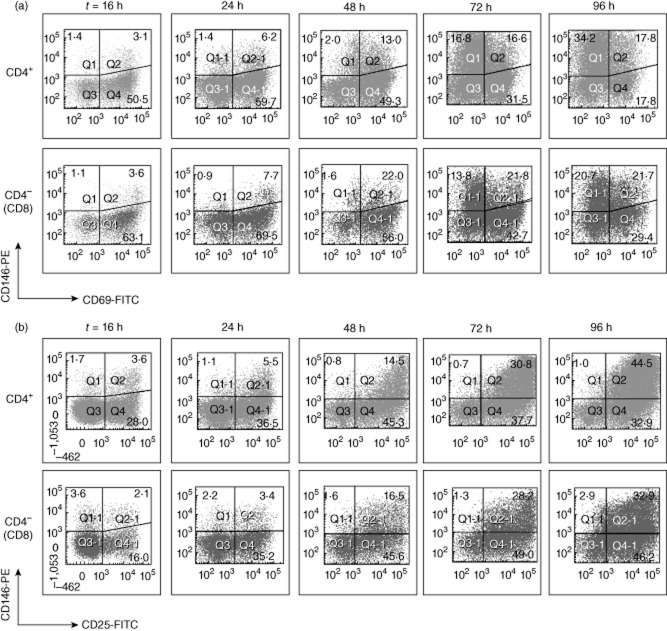
CD4 and CD8 (CD4–) T cells were gated in HD PBMC after exclusion of doublets and gating on intact lymphocytes by light scatter. The strategy is shown in Fig. 1 for a representative sample, analysed ex vivo. Baseline CD146 expression was detected on small percentages of CD4+ and CD4− lymphocytes, but clearly above isotype controls. The cells were stimulated in vitro with anti-CD3 and anti-CD28 antibodies, and subsequent up-regulation of CD146 and activation markers on T cells was measured. Activation was confirmed by up-regulation of CD69 (Fig. 2a) and CD25 (Fig. 2b). CD146 was induced on activated CD4 and CD8 T cells, starting at 24 h and persisting until at least 96 h, similar to CD25. From day 2 onwards, CD146 expression continued to increase, even as activated cells began to lose CD69. T cells underwent blast transformation (not shown), although cell division was not tracked. Thus, both CD4 and CD8 T cells were capable of up-regulating CD146 expression in response to stimulation via CD3 and CD28 in vitro.
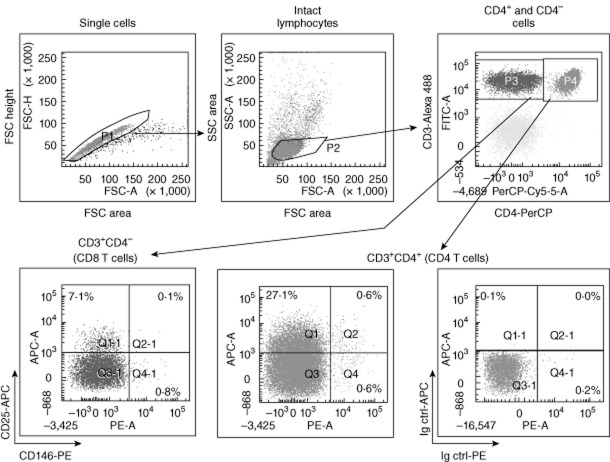
Fig. 1.
Gating strategy for analysis of CD146 expression on T cells. Peripheral blood mononuclear cells (PBMCs) were gated sequentially to exclude doublets and include lymphocytes. T cells were then gated as CD3+ T cells, and usually (except in Fig. 3) divided further into CD4+ and CD4− T cell subsets (the latter representing mainly CD8+ T cells). Both subsets were then analysed for expression of CD146 and an additional marker (CD25 is shown here). The cut-off for positive staining was established using isotype controls (bottom right).
Fig. 2.
CD146 is an activation antigen on CD4 and CD8 T cells in vitro. Peripheral blood mononuclear cells (PBMCs) from a healthy donor were stimulated using plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (0·1 μg/ml) antibodies. After various times, as shown, expression of CD146 and CD69 (a) or CD25 (b) was quantified on CD4 and CD8 T cells (gated as in Fig. 1). Percentages of single- and double-positive cells are shown. Data are representative of five donors. For baseline expression of CD69 and CD146 in healthy donors (HDs), see Figs 3 and 6.
CD146 expression on CD4+ and CD8+ T cells from HDs ex vivo
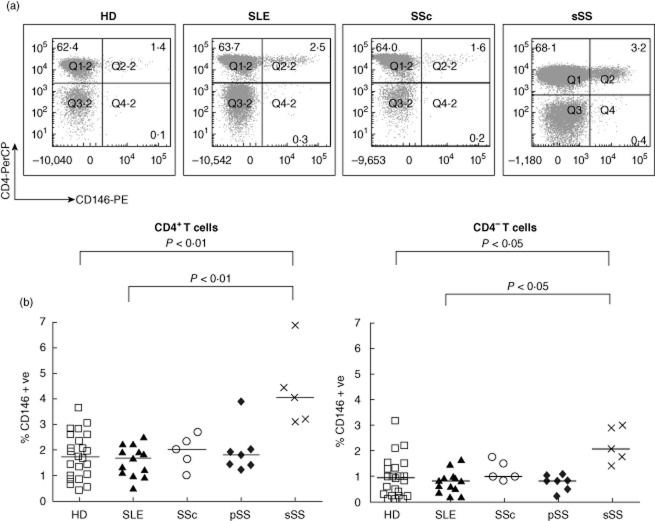
Representative ex-vivo analyses of CD4 versus CD146 staining, gated on CD3+ lymphocytes from HDs, are shown in Fig. 3a. The frequencies of CD146+ cells ranged from 0·3 to 3·6% of CD4+ T cells (Fig. 3b, left; median: 1·70%, IQR: 1·00–2·60%), as reported previously [7]. Within the CD8 (CD4–CD3+) subset, CD146+ T cells were less frequent (Fig. 3b; P < 0·0001 for HD, paired analysis by Wilcoxon test). Isotype control staining did not differ between the T cell subsets (not shown). As described further below, most CTD patients had normal frequencies of CD146+ T cells, but a minority showed increased frequencies.
Fig. 3.
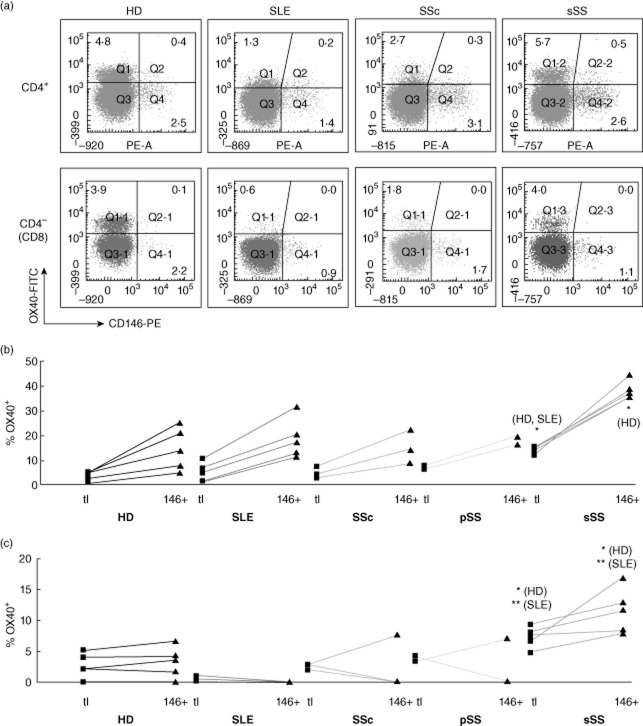
Increased expression of CD146 on circulating T cells in patients with secondary Sjögren's syndrome (sSS) compared to healthy donors (HDs) and other connective tissue diseases (CTD) patients. (a) Representative dot plots of CD146 versus CD4 expression on peripheral blood mononuclear cells (PBMCs) from healthy donors (HD) and patients with systemic lupus erythematosus (SLE), systemic sclerosis (SSc) or sSS after gating for CD3+ T cells. Percentages of single- and double-positive cells are shown. (b) Expression frequency of CD146 within the CD4 and CD8 (CD4–) T cell subsets, calculated from quadrant statistics similar to those shown in (a). Each symbol represents one individual; horizontal bars represent group medians. The increased CD146+ CD4+ T cell frequency in sSS was statistically significant (P-values by Kruskal–Wallis analysis of variance (anova) on ranks with Dunn's post-test for multiple comparisons). In addition, frequencies of CD146+ cells within the CD4 and CD8 subset from each donor were compared by Wilcoxon's signed-rank test (P = 0·0002 for HD, 0·0007 for SLE, 0·016 for pSS; the trend was similar in magnitude for sSS and SSc, but did not reach significance).
Association of CD146 with T cell activation markers in HDs ex vivo
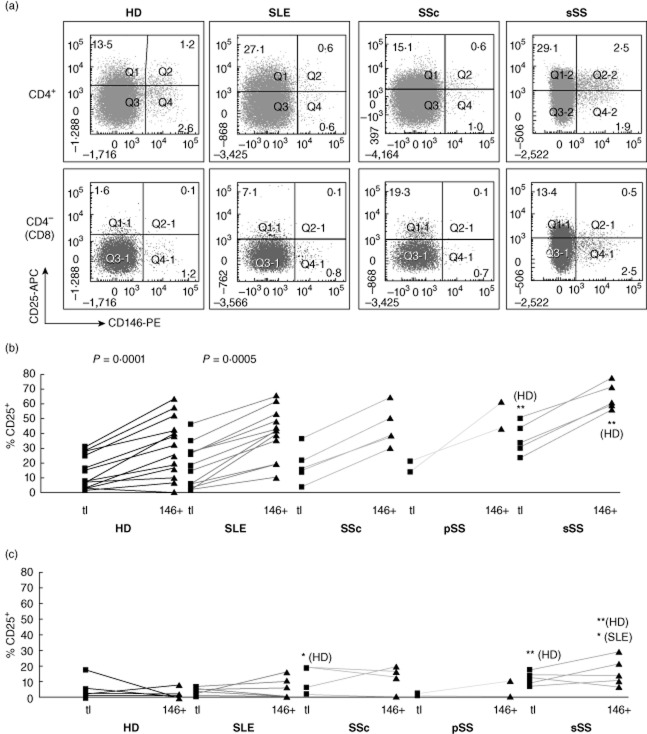
First, we examined whether or not HD T cells expressing CD146 were enriched or depleted of activation markers. Ex vivo, a minority of total CD4+ T cells in HDs expressed CD25 (Fig. 4a, left). The small subset of CD146+ cells appeared to be shifted towards raised CD25 fluorescence intensity compared to the double-negative population, even though most of these cells did not exceed the threshold for positivity. This suggested that most CD146+ T cells express low levels of CD25. If the two markers were expressed independently of each other, the frequency of CD25+ cells in the CD146+ subset should be the same as in bulk CD4 T cells. However, CD25+ cells comprised a greater proportion of CD146+ cells than of total CD4 cells, indicating a mutual association, which was highly reproducible between donors (Fig. 4b, left; numerical P-values indicate a significant association, as assessed by a paired, non-parametric analysis). None the less, many CD25+ cells expressed no detectable CD146. These findings were similar to previous findings in individual donors [7].
Fig. 4.
Co-expression of CD146 with CD25 ex vivo. Co-expression of CD146 with CD25 was analysed in peripheral blood mononuclear cells (PBMCs) from healthy donors (HDs) and connective tissue diseases (CTD) patients. (a) Representative flow cytometry dot-plots of CD146 versus CD25, gated on CD4+ (top row) or CD8 (bottom row) T cells as shown in Fig. 1. Percentages of single- or double-positive cells are shown. (b) In each patient group, expression of CD25 was compared between total CD4 T cells (squares) and CD146+ CD4 T cells (triangles) in individual donors (connected by lines). (c) The same analysis is shown for CD8 T cells. In (b) and (c), the numerical P-values indicate significant (P < 0·05) enrichment for activation marker expression within the CD146+ subset, compared to bulk T cells from the same donor (paired analysis by Wilcoxon's signed-rank test). In contrast, the asterisks indicate significantly increased (*P < 0·05; **P < 0·01, by Kruskal–Wallis analysis of variance (anova) on ranks with Dunn's post-test) frequencies compared to the same population in HDs or disease controls (as indicated in parentheses).
CD25 is expressed on activated effector T cells and, at higher levels, on CD4+ regulatory T cells (Tregs) [23]. Indeed, a small minority of the CD146+CD4+ T cells was CD25high (Fig. 4a, left) and expressed the Treg transcription factor, FoxP3 (Supporting information, Fig. S3). On most CD146+CD4+ T cells, however, CD25 expression was low (Fig. 4a, left). Other T cell activation antigens [OX40 (Fig. 5) and CD69 (Fig. 6), but not major histocompatibility complex (MHC) class II (Supporting information, Fig. S4) or CD70 (Supporting information, Fig. S5)] were also over-represented systematically within the CD146+ population of the CD4+ T cell subset in HDs (a,b, left). (Only a subset of HDs was analysed for all activation markers; the trend for OX40 was consistent but did not reach statistical significance.)
Fig. 5.
Co-expression of CD146 with OX-40 ex vivo. Analysis as in Fig. 4.
Fig. 6.
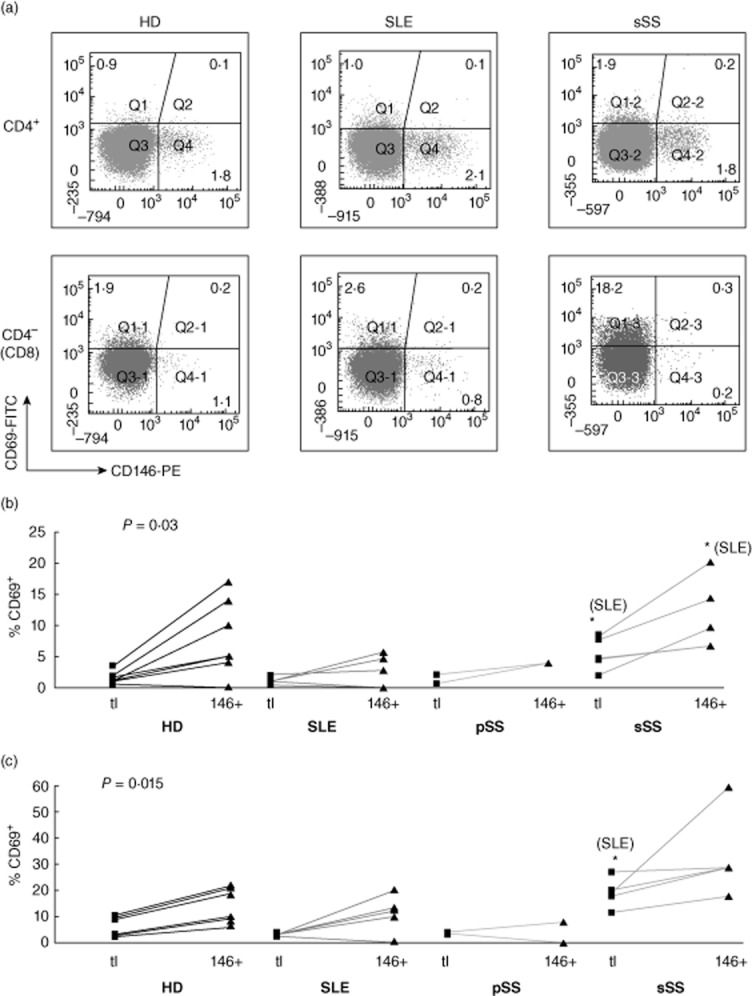
Co-expression of CD146 with CD69 ex vivo. Analysis as in Fig. 4.
Of these activation markers, only CD69 was over-represented consistently in HD CD146+ CD8 cells (Figs 6, Supporting information, Figs S5 and S6, A and C, left panels). Thus, CD146 was associated with recent T cell activation, although none of the markers exhibited perfect overlap and the association was less marked in CD8 cells. In CTD patients, deviations from these normal patterns were uncommon, as described further below.
Correlation of memory and adhesion/homing markers with CD146 expression in HDs
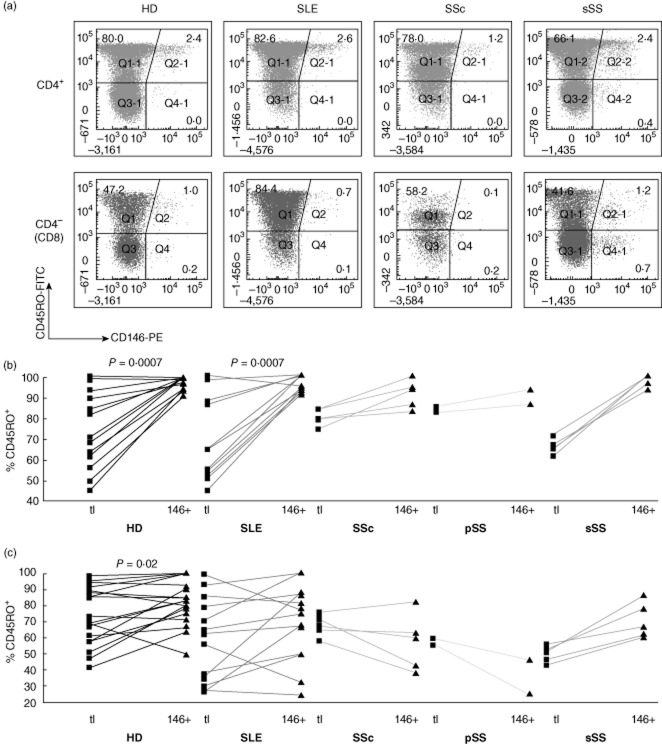
CD45RO is expressed following priming of naive T cells and persists in central and effector memory T cells; chronically stimulated cells may re-express the CD45RA isoform [24,25]. As reported previously in individual donors [7], CD146 expression on HD CD4 T cells was confined to CD45RO+ cells (Fig. 7a,b, left panels). However, within the CD8 subset, both CD45RO+ and RO− cells expressed CD146 (Fig. 7c, left). RO+ cells were enriched among CD146+ CD8 cells, but this trend was far less pronounced than in CD4 cells. CD45RA was analysed in some HDs and patients with SLE, showing reciprocal patterns to those observed with the RO isoform (Supporting information, Fig. S6).
Fig. 7.
Co-expression of CD146 with CD45RO ex vivo. Analysis as in Fig. 4.
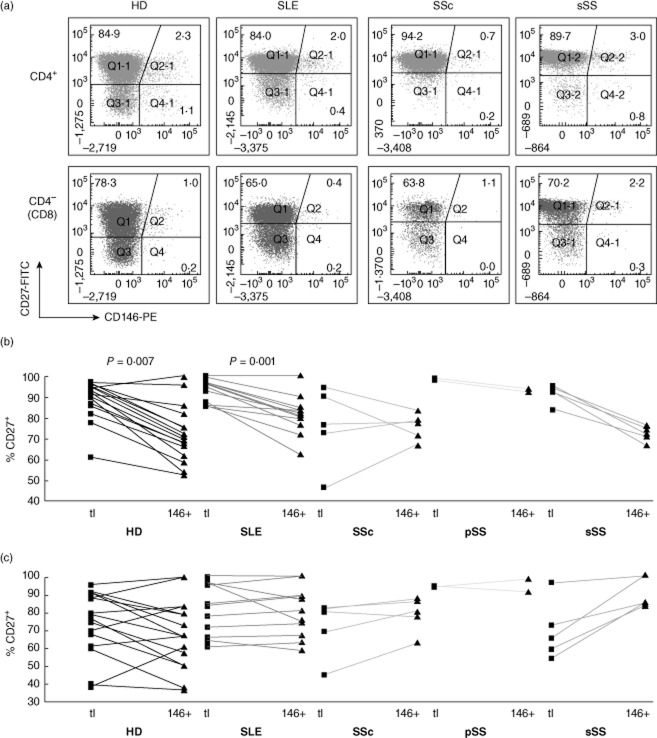
CD27 and CD28 are down-regulated sequentially upon chronic stimulation of T cells [26–28]. CD4+ T cells lacking CD28 have been implicated in atherogenesis [29]; CD28-negative CD8 cell expansion is associated with persistent herpesvirus infections. Both CD27+ and CD27–CD4 and CD8 T cells expressed CD146 (Fig. 8). Within the CD4, but not the CD8 subset, CD146+ cells were enriched for cells that had lost CD27. In most HDs, CD4+CD28− T cells were a small minority; virtually all CD146+ cells retained CD28 expression (Fig. 9). CD28 loss was more extensive in the CD8 subset and CD28–CD146+ CD8 cells were detectable (Fig. 9c), yet CD146 expression on CD8 cells was associated statistically with retention of CD28. In conclusion, CD146 is expressed by both early (CD27+) and late (CD27–) memory/effector CD4 T cells, but not by proatherogenic, ‘senescent’ CD28–CD4+ cells. In contrast, in CD8 cells, CD146 expression may be retained following loss of CD28 in late effector cells.
Fig. 8.
Co-expression of CD146 with CD27 ex vivo. Analysis as in Fig. 4.
Fig. 9.
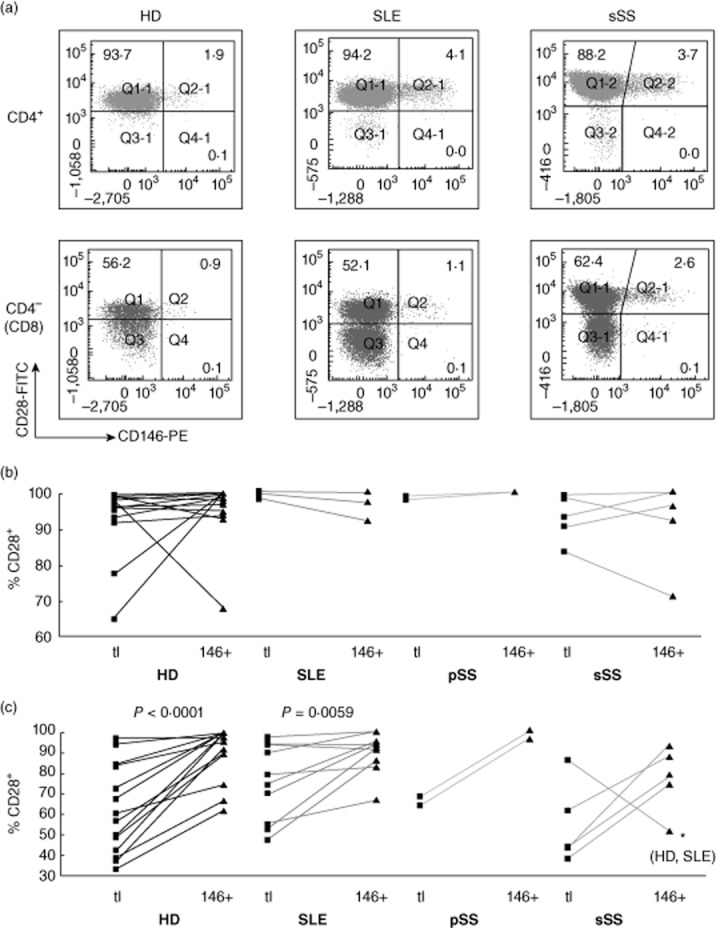
Co-expression of CD146 with CD28 ex vivo. Analysis as in Fig. 4.
Considering the role of CD146 in lymphocyte/endothelial interactions [9], CD146 expression might correlate with adhesion and homing markers. Expression of the proinflammatory chemokine receptor, CCR5, varied between HDs. Within the CD4, but not the CD8 subset, CCR5+ cells were over-represented on CD146+ T cells (Fig. 10). The expression of CXCR3, another chemokine receptor, also varied between donors, independently of CD146 expression (Supporting information, Fig. S7). HD CD4 and CD8 T cells expressed CD31/platelet endothelial cell adhesion molecule (PECAM) (Supporting information, Fig. S8) and CD54/ intercellular adhesion molecule 1 (ICAM-1) (Supporting information, Fig. S9) at varying frequencies. CD146+ HD CD4 T cells, but not CD8 cells, were depleted slightly but systematically of CD31+ cells, and very slightly enriched for CD54+ cells.
Fig. 10.
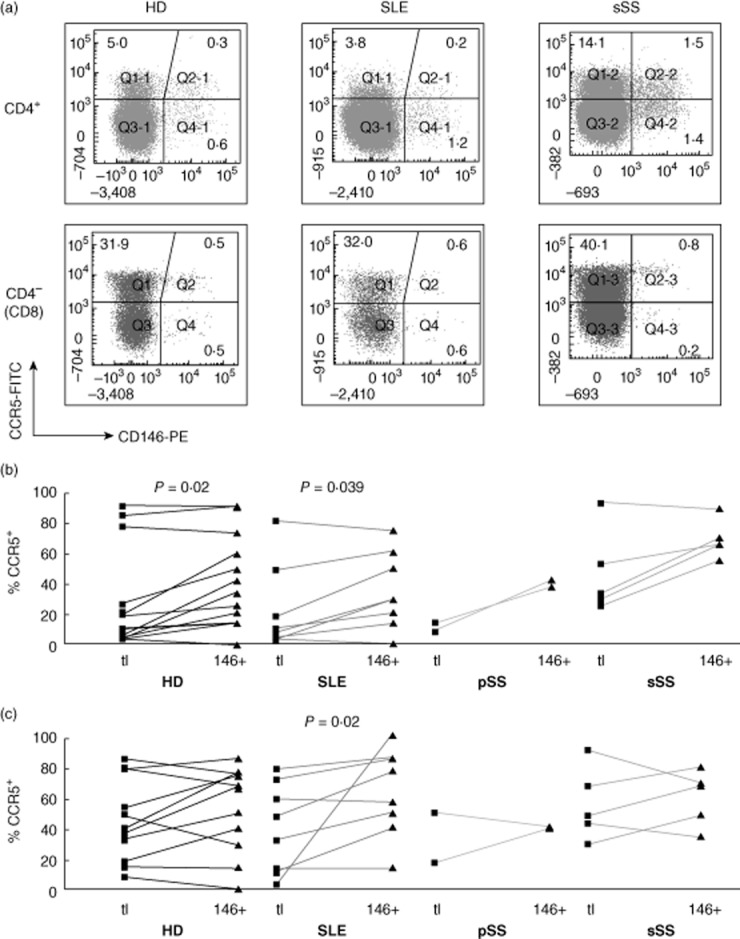
Co-expression of CD146 with CCR5 ex vivo. Analysis as in Fig. 4.
Throughout this study, dead cells were only excluded by scatter; non-specific binding of isotype control antibody to 0·1–0·2% of cells was seen in some experiments (Fig. 1). However, CD4 and CD8 cells differed in their co-expression patterns; some markers were enriched whereas others were depleted, and the associations between CD146 and other markers in CD4+ T cells were consistent between donors and, where previously studied, consistent with earlier work. Taken together, the results are not explained by non-specific staining.
Ex-vivo analysis of CTD patients
Surprisingly few CTD patients showed evidence of CD146 up-regulation ex vivo (Fig. 3). The median frequency of CD146+CD4+ T cells remained normal in patients with SLE (1·60%), SSc (2·0%) and pSS (1·80%; one patient was just above the normal range). In contrast to previously described patients with SLE and pSS [30–32], including patients from our CTD clinic (C. Bryson and F.C. Hall, unpublished data), these patients showed no T cell activation or derangement of memory subsets or adhesion markers (Figs 10 and Supporting information, Figs S4–S9, middle panels). In these patients, systemic T cell dysregulation appeared to be minor or well controlled by therapy. This contrasts with other studies of blood T cell activation in patients with SLE or pSS, with implications for the interpretation of our results (see Discussion).
In contrast, the five sSS patients in our study had significantly increased CD146 expression on CD4 cells (median: 4·0%) and, to a lesser extent, on CD8 cells (Fig. 3). These patients harboured elevated frequencies of CD4 and CD8 cells expressing the activation markers CD25 and OX-40 (Figs 4 and 5; asterisks symbolize significant differences from HDs or other CTD groups by non-parametric anova). Moreover, the correlation of CD146 with activation markers was more extensive in the sSS patients. In all five patients, each of the activation markers tested (CD25, HLA-DQ, OX-40, CD69 and CD70) was over-represented in the CD146+ subpopulation of CD4 cells (Figs 6, Supporting information, Figs S4 and S5). Also, the frequencies of CD25+ and OX40+ in the CD146+ subsets of both CD4 and CD8 cells were increased significantly above healthy donors and SLE disease controls (asterisks). Independently of CD146, the sSS patients exhibited increased CD31 expression on CD4 and CD8 cells; some showed loss of CD28 from CD4 cells (Supporting information, Fig. S8). Other memory, adhesion and homing markers were similar to those in HDs. Thus, circulating T cells in the few CTD patients who exhibited phenotypic T cell activation had increased CD146 expression, associated with a broadened range of activation markers.
Discussion
We examined CD146 expression on circulating CD4 and CD8 T cells of HDs and patients with CTDs, and characterized the relationship of CD146 with surface markers associated with activation, memory, adhesion and homing. As expected, CD146 expression correlated with some activation and memory markers, but unexpected differences between CD4 and CD8 T cells were observed. CD146 on T cells was increased in a small number of patients with sSS, all of whom exhibited systemic T cell activation, but not in patients with other CTDs, who did not.
Previous work has shown CD146 induction by phytohaemagglutinin-activated T cells [3,7]. We found that stimulation of HD T cells with anti-CD3/anti-CD28, a more physiological stimulus, up-regulated CD146 expression with slower kinetics and longer persistence than CD69, but similar to CD25. Both activated CD4 and CD8 T cells expressed CD146. Ex vivo, however, the relationship of CD146 expression to T cell activation was more complex. CD146-expressing CD4 T cells contained a greater proportion of activated-phenotype cells than bulk CD4 T cells (OX40+, CD69+ and low-level CD25 expression). Within the CD4 subset, the CD146+ population comprised almost exclusively CD45RO+/RA–/CD28+ non-senescent memory cells, and was enriched in CD27− cells, suggesting repeated activation. Nevertheless, the correlation with activation was not absolute: most activated cells lacked CD146, and no single marker correlated perfectly with CD146 expression. Thus, CD4 T cell activation in vivo does not induce CD146 expression as uniformly as it does in vitro. This could partly reflect differences in the timing of expression of activation markers post-stimulation but suggests that physiological stimuli induce CD146 expression more selectively than is recapitulated in vitro. A few CD146+CD4 T cells are FoxP3+ CD25high, consistent with a Treg phenotype, but FoxP3 can be expressed by human activated effector T cells and additional markers would be required to address this definitively [33]. Previous work has reported similar findings, albeit with fewer markers analysed in individual donors [7].
Unexpectedly, the association of CD146 with activation and memory ex vivo was less marked in CD8 T cells. In HD CD8 cells, CD146-expressing cells were less frequent than in CD4 cells; of the activation markers studied, only CD69 was enriched significantly in CD146+ CD8 cells. Moreover, CD146 expression was found both on CD8 cells expressing and lacking CD45RO, CD45RA, CD27 and CD28. Late (CD45RA+CD28–) effector CD8 cells express CD146. Collectively, these findings suggest two modes of CD146 expression: one that is related closely to recent or chronic memory T cell activation and predominates in healthy donor CD4 T cells, and another, which appears to be more stochastic and predominates in the CD8 subset.
Consistent with previous reports [11], circulating T cells in patients with sSS were phenotypically activated (increased CD25, OX40, and perhaps CD69), both in the CD4 and the CD8 subset. The increased frequency of CD146-expressing CD4 and CD8 cells in these patients, as well as the correlation with several activation markers, is consistent with this. Combinatorial analysis of activation markers including CD146 may improve the assessment of T cell activation in CTDs. Importantly, CTD patients in general maintain normal or slightly reduced lymphocyte counts in blood [10,11]; PBMC yields (by haemocytometer counting) were not markedly abnormal in our CTD patients.
Unexpectedly, activation markers were not increased in T cells from our SLE and most pSS patients. This contrasted with previous studies, in which increased frequencies of recently and chronically activated and senescent T cells were found in patients with SLE [10] or pSS [34–37], including patients studied by us (C. Bryson, F.C. Hall, unpublished observations). Most of the patients examined in the present study lacked critical organ involvement and had mild or moderate disease activity. Their disease was well controlled by drug therapy, ranging from hydroxychloroquine alone to various combinations of anti-proliferative agents, corticosteroids and biologicals (Supporting information, Table S1). This might account for their non-activated peripheral T cell phenotypes and low CD146 expression. This is not a sensitivity issue, as we detected T cell activation and CD146 up-regulation in sSS, and more recently in a separate study of patients with inflammatory arthritis, using the same reagents and protocols (C. Wu, R. Busch, J.S.H. Gaston, unpublished data). As a result of the unexpected non-activated phenotypes in these patients, this study cannot address whether CD146 up-regulation is a disease-specific feature of sSS or a consequence of systemic hyperactivity, which happened to be detectable only in sSS patients in our study. The latter explanation is, however, both more conservative and plausible. A much larger multivariate analysis of CTD patients with diverse diagnoses, varying in T cell activation, would be required to address this fully and to account for confounding variables.
Our unpublished work (C. Wu et al.) also confirms previous findings (cf. Introduction) that CD146+ CD4 cells are strongly enriched for Th17 cells [CCR6+, CD161+; mitogen-stimulated interleukin (IL)-17 and IL-22 secretion]. Even though the present study was not designed to explore cytokine secretion patterns, our findings might suggest that Th17 activity in these CTD patients was well controlled by standard therapy.
The increased T cell activation and CD146 expression in our sSS patients was not explained by unique features with regard to disease activity, serology or severity of immunosuppression, compared to the other patient groups (Supporting information, Table S1). T cell hyperactivity may be inherently greater in sSS, or more difficult to control with drugs, relating possibly to more extensive organ involvement than would be present in pSS, for example. However, other clinical variables, rather than their diagnosis of sSS, might have been critical. In any case, combinatorial analysis of T cell activation markers and CD146 could aid differentiation between patient subgroups on a clinical spectrum of CTD. Future studies will show whether this might identify subpopulations of CTD patients who would benefit from more aggressive therapy, or from targeting Th17 cells specifically.
Effector lymphocyte subsets are recruited to inflammatory sites by several mechanisms. T cell recruitment by CCL21 and its receptor, CCR7, promotes ectopic lymphoneogenesis at inflammatory lesions in subsets of patients with Sjögren's syndrome and SLE [38–40]. Another pathway recruits effector T cells via other, proinflammatory chemokines and their receptors, including CCR5 [41]. The correlation between CD146 and CCR5 on T cells suggests that CD146 participates in the latter pathway, and this may be exaggerated in our sSS patients. This is consistent with increased CD146 expression by tissue-infiltrating T cells (see Introduction).
One study reported that the frequency of circulating CD146+ apoptotic cells was elevated in SLE, correlating with endothelial dysfunction, a known risk factor for atherogenesis and cardiovascular morbidity [42]. Endothelial cells were enumerated by staining for CD146, but lymphocytes were not excluded. However, circulating endothelial cells (defined by CD146 and other endothelial antigens and absence of leukocyte markers [43]) are vastly outnumbered by CD146+ lymphocytes, which might have confounded these results [7] (Supporting information, Fig. S10). The possibility remained that CD146 might identify a pro-atherogenic T cell subset. However, we observed no increase in the frequency of CD146+ T cells in SLE, even though atherosclerosis is accelerated in this disease [12,44,45]; nor did we find unusual patterns of CD146 expression on T cells in HDs with a history of CVD. T cells in atherosclerotic plaque are CD4+CD28–, and an increased frequency of such cells in blood correlates with atherosclerosis [18,46], yet we found no correlation of CD28 down-regulation with CD146 expression. T cells in atherosclerotic plaque express CCR5 [47–50], and this marker was associated weakly with CD146 expression; however, CCR5 also directs homing to other inflamed tissues and to the gastrointestinal tract. Collectively, our work provides no support for CD146 as a marker of atherogenesis.
In summary, our studies confirm the status of CD146 as an activation-related antigen on T cells. Ex vivo, CD146 expression was correlated with circulating, non-senescent (CD28+CD45RO+) early and late (CD27+ or CD27–) memory CD4 T cells. CD146 expression in CD4 cells was associated with recent activation, albeit less closely than in vitro, and was found with increased frequency in patients with sSS, who exhibited phenotypic T cell hyperactivity despite immunomodulatory therapy. On CD8 T cells, CD146 expression extended to CD28− late effector cells, but the association with activation was limited, except in patients with CD8 cell hyperactivity. CD146 expression was associated weakly with CCR5, but not with other adhesion or homing markers. Moreover, our studies show heterogeneity with regard to residual systemic T cell hyperactivity (including CD146 expression) among conventionally treated patients with CTDs. This might be more prominent, or less well controlled, by drug therapy in particular patients, who might therefore benefit from additional T cell-targeted therapy.
Acknowledgments
This work was supported by a summer studentship from the Pathological Society of Great Britain and Ireland awarded to A.V.H. and by funding from Actelion Pharmaceuticals and from the Cambridge Biomedical Research Centre of the National Institute for Health Research, both to F.C.H. R.B. was funded by Senior Research Fellowships from the Elmore Fund at Sidney Sussex College and Arthritis Research UK (ref. 18543). We thank Michael Bacon for technical assistance, Drs Kaisa Mäki-Petäjä and Ian Wilkinson for referring healthy donors to the study and J.S.H. Gaston and W.-F. Ng for helpful discussions.
Disclosure
The authors disclose no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Similar patterns of CD146 co-expression with other markers after distinguishing CD3+ T cell subsets by either CD4 or CD8 staining. Peripheral blood mononuclear cells (PBMCs) from a systemic lupus erythematosus (SLE) patient were stained for CD146 and a panel other markers (‘Antigen X’). (a) CD4 T cells were gated either as CD3+CD4+ or CD3+CD8− lymphocytes. Frequencies of CD146+ CD4 cells with or without Antigen X were then enumerated. (b) The same analysis performed for CD8 T cells, which were gated either as CD3+CD4− or CD3+CD8+ lymphocytes. In both subsets, closely similar expression patterns were obtained with either gating procedure.
Fig. S2. No effect of cryopreservation on patterns of CD146 versus CD45RO expression on T cells. Analysis of three systemic lupus erythematosus (SLE) patients. (a) Representative dot-plots from one patient, gated on CD4+ or CD4− T cells. (b) Percentages of indicated subpopulations in three patients. The CD4+/CD4− ratio was also unaffected by cryopreservation.
Fig. S3. Surface CD146 versus intracellular forkhead box protein 3 (FoxP3) expression in gated CD4+ and CD8 peripheral blood T cells from a representative HD (of five analysed).
Fig. S4. CD146 versus human leucocyte antigen D-related (HLA-DQ) [major histocompatibility complex (MHC) class II] expression in peripheral blood CD4+ and CD4− (CD8) T cells from healthy donors (HDs) and connective tissue disease (CTD) patients, analysed as in Fig. 4 of the main paper.
Fig. S5. CD146 versus CD70 expression, analysed as in Fig. 4 of the main paper.
Fig. S6. CD146 versus CD45RA expression in T cells from healthy donors (HDs) and systemic lupus erythematosus (SLE) patients, analysed as in Fig. 4 of the main paper. #Indicates a single donor in whom carryover of CD3− antigen-presenting cells (APC) from an adjacent well caused 100% of T cells to be aberrantly positive for CD146.
Fig. S7. CXCR3 expression in total versus CD146+ CD4 and CD8 T cells from healthy donors (HDs) and systemic lupus erythematosus (SLE) patients; paired analysis as in Fig. 4b,c of the main paper (P > 0·05, not significant).
Fig. S8. CD146 versus CD31 expression, analysed as in Fig. 4 of the main paper.
Fig. S9. CD146 versus CD54/intercellular adhesion molecule 1 (ICAM-1) expression, analysed in healthy donors (HDs) and systemic lupus erythematosus (SLE) patients, as in Fig. 4 of the main paper.
Fig. S10. CD146+ lymphocytes greatly outnumber CD146+ circulating endothelial cells. Peripheral blood mononuclear cells (PBMCs) from a healthy donor were co-stained for CD45 (leucocyte common antigen), CD146 and CD34 (the latter is expressed both on haematopoietic progenitors and on endothelial cells). Numbers represent percentages or frequencies. In the CD45+ leucocyte gate a proportion of cells stained for either CD146 or CD34, but not both. In the CD45− gate, a small number of CD34+CD146+ double-positive events were detected, which may be circulating endothelial cells (versus one event detected in isotype control).
Table S1. Clinical characteristics of patients.
References
- 1.Bardin N, Frances V, Lesaule G, Horschowski N, George F, Sampol J. Identification of the S-Endo 1 endothelial-associated antigen. Biochem Biophys Res Commun. 1996;218:210–216. doi: 10.1006/bbrc.1996.0037. [DOI] [PubMed] [Google Scholar]
- 2.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Pickl WF, Majdic O, Fischer GF, et al. MUC18/MCAM (CD146), an activation antigen of human T lymphocytes. J Immunol. 1997;158:2107–2115. [PubMed] [Google Scholar]
- 4.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(Pt 12):3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 5.Dagur PK, Tatlici G, Gourley M, et al. CD146+ T lymphocytes are increased in both the peripheral circulation and in the synovial effusions of patients with various musculoskeletal diseases and display pro-inflammatory gene profiles. Cytometry B Clin Cytom. 2010;78:88–95. doi: 10.1002/cyto.b.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP., Jr CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood. 2005;106:2923–2924. doi: 10.1182/blood-2005-06-2307. [DOI] [PubMed] [Google Scholar]
- 7.Elshal MF, Khan SS, Raghavachari N, et al. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC Immunol. 2007;8:29. doi: 10.1186/1471-2172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Despoix N, Walzer T, Jouve N, et al. Mouse CD146/MCAM is a marker of natural killer cell maturation. Eur J Immunol. 2008;38:2855–2864. doi: 10.1002/eji.200838469. [DOI] [PubMed] [Google Scholar]
- 9.Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J Immunol. 2007;179:6673–6685. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- 10.Bryson C, Busch R, Hall FC. T-cell dysregulation in systemic lupus erythematosus: pathophysiology and opportunities for biomarker development. Curr Rheumatol Rev. 2008;4:23–33. [Google Scholar]
- 11.Busch R, Hadjinicolaou AV, Hall FC. Local activation and systemic dysregulation of T lymphocytes in Sjögren's syndrome. Curr Pharm Biotechnol. 2012;13:2009–2021. doi: 10.2174/138920112802273092. [DOI] [PubMed] [Google Scholar]
- 12.Hall FC, Dalbeth N. Disease modification and cardiovascular risk reduction: two sides of the same coin? Rheumatology (Oxf) 2005;44:1473–1482. doi: 10.1093/rheumatology/kei012. [DOI] [PubMed] [Google Scholar]
- 13.Vaudo G, Bocci EB, Shoenfeld Y, et al. Precocious intima-media thickening in patients with primary Sjogren's syndrome. Arthritis Rheum. 2005;52:3890–3897. doi: 10.1002/art.21475. [DOI] [PubMed] [Google Scholar]
- 14.Gerli R, Vaudo G, Bocci EB, et al. Functional impairment of the arterial wall in primary Sjogren's syndrome: combined action of immunologic and inflammatory factors. Arthritis Care Res (Hoboken) 2010;62:712–718. doi: 10.1002/acr.20117. [DOI] [PubMed] [Google Scholar]
- 15.Soltesz P, Bereczki D, Szodoray P, et al. Endothelial cell markers reflecting endothelial cell dysfunction in patients with mixed connective tissue disease. Arthritis Res Ther. 2010;12:R78. doi: 10.1186/ar2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vegh J, Szodoray P, Kappelmayer J, et al. Clinical and immunoserological characteristics of mixed connective tissue disease associated with pulmonary arterial hypertension. Scand J Immunol. 2006;64:69–76. doi: 10.1111/j.1365-3083.2006.01770.x. [DOI] [PubMed] [Google Scholar]
- 17.Fleming JN, Nash RA, Mahoney WM, Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep. 2009;11:103–110. doi: 10.1007/s11926-009-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr Opin Pharmacol. 2010;10:197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Nadashkevich O, Davis P, Fritzler MJ. Revising the classification criteria for systemic sclerosis. Arthritis Rheum. 2006;55:992–993. doi: 10.1002/art.22364. [DOI] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 24.Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 25.Clement LT. Isoforms of the CD45 common leukocyte antigen family: markers for human T-cell differentiation. J Clin Immunol. 1992;12:1–10. doi: 10.1007/BF00918266. [DOI] [PubMed] [Google Scholar]
- 26.Fritsch RD, Shen X, Illei GG, et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2184–2197. doi: 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- 27.Weng NP, Akbar AN, Goronzy J. CD28(–) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 29.Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009;81:11–19. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- 30.Neidhart M, Pataki F, Michel BA, Fehr K. CD45 isoforms expression on CD4+ and CD8+ peripheral blood T-lymphocytes is related to auto-immune processes and hematological manifestations in systemic lupus erythematosus. Schweiz Med Wochenschr. 1996;126:1922–1925. [PubMed] [Google Scholar]
- 31.Gordon C, Matthews N, Schlesinger BC, et al. Active systemic lupus erythematosus is associated with the recruitment of naive/resting T cells. Br J Rheumatol. 1996;35:226–230. doi: 10.1093/rheumatology/35.3.226. [DOI] [PubMed] [Google Scholar]
- 32.McKinney EF, Lyons PA, Carr EJ, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–591. doi: 10.1038/nm.2130. 1p following 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur G, Busch R, Gaston JSH. The role of FoxP3 in regulatory T cell differentiation and function. Curr Immunol Rev. 2009;5:89–101. [Google Scholar]
- 34.Celenligil H, Kansu E, Ruacan S, Eratalay K, Irkec M. Characterization of peripheral blood and salivary gland lymphocytes in Sjogren's syndrome. Oral Surg Oral Med Oral Pathol. 1990;69:572–577. doi: 10.1016/0030-4220(90)90238-n. [DOI] [PubMed] [Google Scholar]
- 35.Fauchais AL, Boumediene A, Lalloue F, et al. Brain-derived neurotrophic factor and nerve growth factor correlate with T-cell activation in primary Sjogren's syndrome. Scand J Rheumatol. 2009;38:50–57. doi: 10.1080/03009740802378832. [DOI] [PubMed] [Google Scholar]
- 36.Szodoray P, Gal I, Barath S, et al. Immunological alterations in newly diagnosed primary Sjogren's syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand J Rheumatol. 2008;37:205–212. doi: 10.1080/03009740801910361. [DOI] [PubMed] [Google Scholar]
- 37.Youinou P, Pennec YL, Blaschek MA, et al. Activation of peripheral blood lymphocytes in patients with primary Sjogren's syndrome. Rheumatol Int. 1988;8:125–130. doi: 10.1007/BF00272434. [DOI] [PubMed] [Google Scholar]
- 38.Barone F, Bombardieri M, Rosado MM, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–5140. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 39.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. ‘Lymphoid’ chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjogren's syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–418. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Barone F, Bombardieri M, Manzo A, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren's syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 41.Petrek M, Cermakova Z, Hutyrova B, et al. CC chemokine receptor 5 and interleukin-1 receptor antagonist gene polymorphisms in patients with primary Sjogren's syndrome. Clin Exp Rheumatol. 2002;20:701–703. [PubMed] [Google Scholar]
- 42.Rajagopalan S, Somers EC, Brook RD, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 43.Goon PK, Boos CJ, Stonelake PS, Blann AD, Lip GY. Detection and quantification of mature circulating endothelial cells using flow cytometry and immunomagnetic beads: a methodological comparison. Thromb Haemost. 2006;96:45–52. doi: 10.1160/TH06-04-0185. [DOI] [PubMed] [Google Scholar]
- 44.Frostegard J. Systemic lupus erythematosus and cardiovascular disease. Lupus. 2008;17:364–367. doi: 10.1177/0961203308089988. [DOI] [PubMed] [Google Scholar]
- 45.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10:224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowik M, Nowacki P, Grabarek J, et al. Can we talk about CD4+CD28− lymphocytes as a risk factor for ischemic stroke? Eur Neurol. 2007;58:26–33. doi: 10.1159/000102163. [DOI] [PubMed] [Google Scholar]
- 47.Braunersreuther V, Zernecke A, Arnaud C, et al. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 48.Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–149. doi: 10.1097/00041433-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson C, Eneslatt K, Ivanoff J, Rantapaa-Dahlqvist S, Sundqvist KG. Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus. 2003;12:766–774. doi: 10.1191/0961203303lu467oa. [DOI] [PubMed] [Google Scholar]
- 50.Quinones MP, Martinez HG, Jimenez F, et al. CC chemokine receptor 5 influences late-stage atherosclerosis. Atherosclerosis. 2007;195:e92–103. doi: 10.1016/j.atherosclerosis.2007.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.