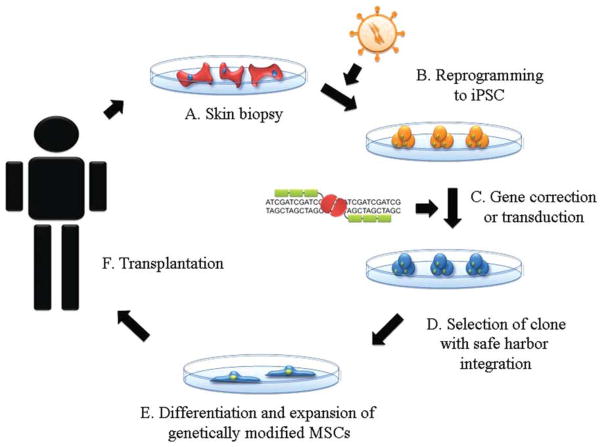
Figure 1.
Schematic diagram of iPSC therapy. (A): Fibroblasts from skin biopsy are cultured from patients. (B): Patient-specific cells can be reprogrammed by viral delivery of induction factors or nonintegrating methods. (C): Gene correction can be accomplished by vector-mediated gene transfer or gene exchange by homologous recombination. (D): Gene-corrected iPSCs can be screened by sequencing to find a clone with proper gene correction or integration into a safe harbor site. (E): Gene-modified iPSCs can be differentiated into MSCs and expanded. (F): MSCs with integration into the controlled site can be tested, expanded, and purified in a good manufacturing practice facility and could then be transplanted to the patient, following appropriate clearance by all regulatory agencies. Abbreviations: iPSC, induced pluripotent stem cell; MSCs, mesenchymal stem cells.