Abstract
The innate immune system is comprised of cellular sentinels that often serve as the first responders to injury and invading pathogens. Our basic understanding of innate immunity is derived from research conducted in peripheral lymphoid tissues. However, it is now recognized that most non-lymphoid tissues throughout the body are equipped with specialized innate immune cells that are uniquely adapted to the niches in which they reside. The central nervous system (CNS) is a particularly interesting compartment because it contains a population of post-mitotic cells (neurons) that are intolerant of robust, cytopathic inflammatory responses observed in many peripheral tissues. Thus, evolutionary adaptations have fitted the CNS with a unique array of innate immune sentinels that facilitate the development of local inflammatory responses but attempt to do so in a manner that preserves the integrity of its post-mitotic residents. Interestingly, studies have even suggested that CNS resident innate immune cells contribute to the homeostasis of this compartment and promote neural activity. In this review we discuss recent advances in our understanding of CNS innate immune sentinels and how novel imaging approaches such as intravital two-photon laser scanning microscopy (TPLSM) have shed light on these cells during states of health and disease.
Keywords: microglia, dendritic cells, macrophages, CNS, innate immunity
Origin and Anatomy of CNS Innate Immune Cells
The adult CNS is inhabited by several innate immune cells that are derived from primitive myeloid precursors or hematopoietic cells. The lineage derivation of these cells is discussed extensively in several recent reviews1-3 and thus will only be mentioned briefly here. The most abundant CNS innate immune sentinels reside in the parenchyma and are referred to as microglia. It was originally thought that microglia were bone marrow-derived; however, recent studies have elegantly shown that these cells come from primitive yolk sac progenitors and can be maintained in the parenchyma for life without replenishment from the bone marrow.4 In mice, primitive macrophages appear in the embryonic (E) yolk sac at E7.5, differentiate into microglia and colonize the CNS before vascularization of the embryo occurs. Thus, microglia share many features with macrophages, but are nevertheless uniquely adapted to function in the CNS parenchyma. For example, microglia have small cell bodies from which many ramifications (or branches) emanate (Fig. 1A and D; Vid. S1). These branches are highly dynamic and continually scan the parenchyma (Vid. S1).5 From end to end, microglia can span distances of 50 to 100 microns, and it is estimated that collectively these cells survey the entire parenchymal space every 2–3 h.5
Figure 1.
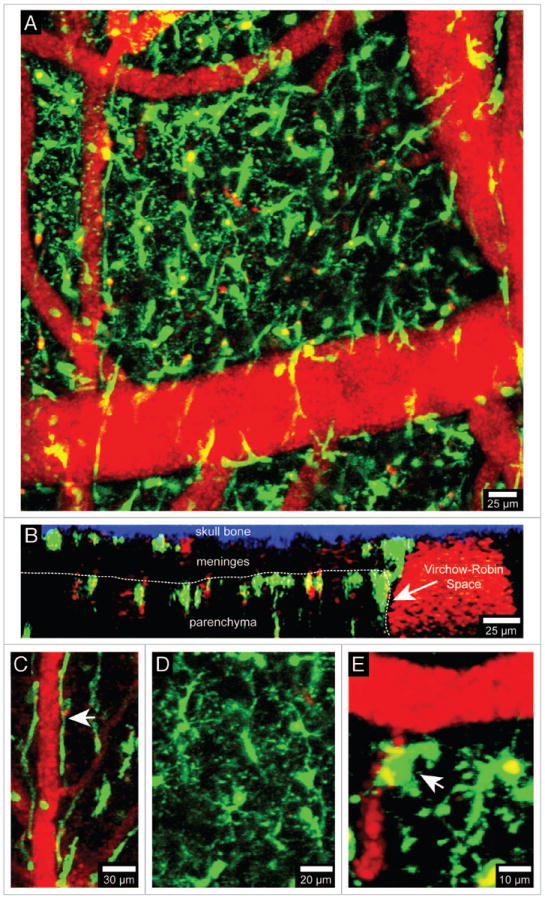
Visualization of brain myeloid cells in CX3CR1-GFP mice. TPLSM was used to capture 4D time-lapses through a thinned skull window of a naïve CX3CR1-GFP+/- mouse. The bone was thinned down manually to a thickness of ~30 μm and then imaged using a Leica SP5 two-photon microscope fitted with a 20x water dipping objective (1.0 NA). For imaging, the lens was dipped into artificial cerebral spinal fluid placed on top of the thinned skull window. Images were collected with a 1.0 μm step size to a depth of 100 μm beneath the skull surface. Z stacks were acquired every minute. Panel A shows the xy distribution of innate myeloid cells (green) in the meninges and neocortex of a naïve mouse brain. Blood vessels (red) were labeled by injecting 655-nm quantum dots intravenously before imaging. Panel B shows an xz projection from the same image stack in which the skull bone (blue), meninges, and brain parenchyma are visible. The white dotted line denotes the glial limitans. The Virchow-Robin space adjacent to a large blood vessel is also visible (white arrow). Note that the density of innate myeloid cells is greater in the parenchyma than in the meninges. CX3CR1-GFP+/- mice can be used to visualize meningeal macrophages (C, white arrow), microglia (D), and perivascular macrophages (E, white arrow). Meningeal macrophages (C) are worm-like cells that line blood vessels in the meninges. Perivascular macrophages (E) reside in Virchow-Robin spaces and adjacent to blood vessels found the brain parenchyma (white arrow). Microglia (D) are the most common CNS myeloid cell and are distributed uniformly throughout the brain parenchyma. Note that microglia are highly ramified cells, whereas meningeal and perivascular macrophages are not. See Video S1.
In contrast to microglia, all other innate immune sentinels residing in the normal CNS are hematopoietically-derived.1-3 Most of these cells are of a myeloid lineage and can be visualized using CX3CR1-green fluorescent protein (GFP)6 and lysozyme M-GFP (LysM-GFP)7 mice (Figs. 1 and 2). The CNS is bathed in a fluid referred to as cerebral spinal fluid (CSF), which is generated by the choroid plexus. CSF is continually pumped throughout the ventricles and lining of the CNS (or meninges), and these structures are anatomically distinct from the CNS parenchyma. The meninges, choroid plexus, and perivascular spaces are inhabited by specialized myeloid sentinels that are aptly named meningeal, choroid plexus, and perivascular macrophages, respectively.1 Meningeal macrophages are long, worm-like cells that sit along meningeal vessels (Figs. 1A–C and 2A–C; Vids. S1 and S2). These cells, visible in CX3CR1-GFP and LysM-GFP reporter mice, are stationary in the uninflamed brain but highly dynamic; their protrusions constantly scan the space around meningeal blood vessels (Vids. S1 and S2). Perivascular macrophages are sentinels that inhabit the spaces around blood vessels which penetrate the brain parenchyma. The macrophages residing in this space have a more compact, amoeboid shape (Figs. 1A, 1E, 2A and 2E) and continually scan their perivascular niche similar to meningeal macrophages (Vids. S1 and S2). Lastly, a specialized population of macrophages resides in the choroid plexus. This anatomical region is particularly vulnerable to peripheral insults because it contains fenestrated blood vessels (most other vessels in the CNS are non-fenestrated). Thus, it is important to have innate immune sentinels monitor this specialized anatomical region. To date, choroid plexus macrophages have not been imaged in four dimensions (4D), but it is likely that these cells possess dynamic scanning properties similar to meningeal and perivascular macrophages.
Figure 2.
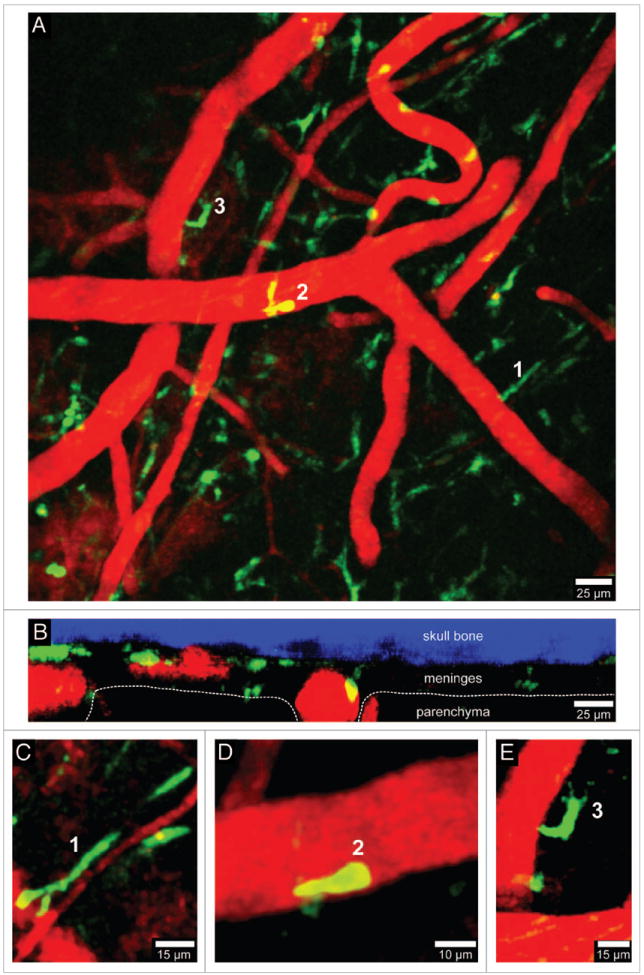
Visualization of peripherally-derived myelomonocytic cells in LysM-GFP mice. A 3D time-lapse was captured through a thinned skull window of a naïve LysM-GFP mouse in a manner similar to that described in Figure 1. Monocytes, macrophages, and neutrophils (but not microglia) are visible in this transgenic mouse strain. Panel A (xy maximal projection) shows the distribution of myelomonocytic cells (green) in relation to blood vessels (red). Note in B (xz projection) that the LysM-GFP+ cells reside exclusively in the meningeal and perivascular spaces. Three cellular morphologies are depicted in panels C–E (labeled 1–3). Worm-like meningeal macrophages (C) are visible along meningeal blood vessels similar to those seen in CX3CR1-GFP+/- mice. On occasion, neutrophils / monocytes (D) can be observed patrolling blood vessels. In addition, amoeboid cells (E) are also visible around blood vessels (possibly perivascular macrophages). Skull bone is shown in blue. See Video S2.
CNS resident microglia and macrophages are all classified as antigen presenting cells (APCs) given their ability to present peptides in MHC class II complexes; however, microglia often considered poor antigen presenters relative to their bone marrow-derived myeloid counterparts.8,9 This conclusion is based primarily on ex vivo stimulation assays in which APCs are extracted from the brain and mixed with antigen-specific T cells. It remains to be determined whether a similar conclusion will be drawn when CNS APCs are studied intravitally. While microglia and macrophages have the capacity to present antigen, dendritic cells (DCs) are the most potent APCs in the body. The brain was once thought devoid of DCs, but recent studies have revealed that the meninges and choroid plexus contain a population of CD11c-expressing cells that are bone marrow-derived, FMS-like receptor tyrosine kinase 3 (Flt3) ligand responsive, and have a 5–7 d half-life.10 In addition, genomic analyses demonstrated that these cells are similar to splenic DCs.10 CD11c-YFP reporter mice11 are commonly used to define the anatomy of DCs in the resting brain (Fig. 3; Vid. S3).12,13 CD11c-YFP+ cells are sparsely distributed in the meningeal space and look morphologically different than worm-like meningeal macrophages (Figs. 1C and 2C; Vids. S1 and S2). CD11c-expressing cells can also be found in perivascular spaces and occasionally the brain parenchyma itself.12,13 While CD11c is often used as a marker to define DCs in peripheral tissues, it is unclear whether all CD11c-expressing cells in the CNS represent bona fide DCs or rather a mix of innate immune cells (the latter is more likely). For example, microglia are not DCs but are known to upregulate CD11c upon activation,9,14,15 and a small fraction even express CD11c in the resting brain.12,13 Thus, CNS DCs should be not identified on the basis of CD11c expression alone. Nevertheless, it is clear that the concept of the CNS being devoid of professional APCs must be revised. This compartment contains an elaborate sentinel program with cells poised to detect damage, infections, etc. in all relevant anatomical positions.
Figure 3.
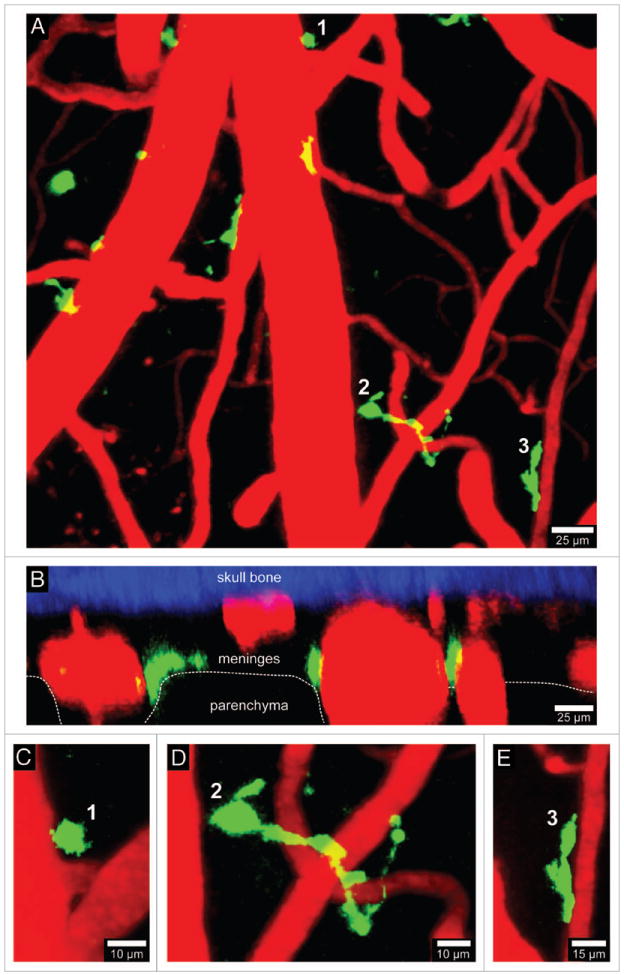
Visualization of CD11c-YFP+ APCs. A 3D time-lapse was captured through a thinned skull window of naïve CD11c-YFP mouse in a manner similar to that described in Figure 1. The majority of CD11c-YFP+ cells (green) visible through a thinned skull reside in the meninges and perivascular spaces. Panels A (xy projection) and B (xz projection) show that CD11c-YFP+ cells are sparsely distributed in the meninges and perivascular spaces of a naïve mouse. There are three distinct cellular morphologies depicted in panels C–E (labeled 1–3). Small spheroid cells (C) that resemble monocytes are visible around blood vessels. There are also long stringy cells 50–75 μm in length (D) that are not completely juxtaposed to the vasculature but are instead intertwined with vessels. Lastly, juxtavascular CD11c-YFP+ cells (E) are visible that share some similarities (e.g., amoeboid) to the perivasular macrophages seen in LysM-GFP mice. Skull bone is shown in blue. See Video S3.
CNS Imaging
Noninvasive visualization techniques involving Doppler ultrasound, computed tomography (CT), positron electron tomography (PET), and magnetic resonance imaging (MRI) have allowed precise mapping of neuroanatomical structures and are commonly used in clinical settings to facilitate diagnosis of CNS disorders. However, these techniques do not provide the spatial and temporal resolution required to monitor the dynamics of innate immune sentinels in the CNS during states of health and disease. To advance our understanding of cellular dynamics, researchers have utilized advanced light microscopy approaches such as time-lapse confocal microscopy and two-photon laser scanning microscopy (TPLSM) in combination with fluorophores/fluorescent proteins to image the CNS in 4D.16,17 Five years following the development of TPLSM,18 Yuste and colleagues first reported the imaging of calcium dynamics in dendritic spines of rat hippocampal neurons in brain slice cultures.19 Since then, remarkable technological improvements in TPLSM have coincided with exciting new discoveries in the fields of neuroscience and neuroimmunology.5,19-28
TPSLM is an optical imaging approach that relies on fluorescence emission detection.18 Early TPLSM studies utilized fluorescent labeling techniques such as Ca2+ indicator dyes, immunohistochemical stains, etc.29,30 In 1996, shortly after green fluorescent protein (GFP) was successfully cloned from the jellyfish Aequorea victoria, Potter et al. conducted the first TPSLM imaging of GFP in developing optic ganglia of Drosophila.31 Since then, fluorescent proteins derived from jellyfish and corals have become mainstream tools in the TPLSM community.17,32 Use of TPSLM to advance our understanding of the nervous system in mammals began with the imaging of brain slice cultures.33 This approach was then quickly adapted to anesthetized rodents which represented an “intravital” preparation that revolutionized the field of neuroscience by allowing researchers to visualize the dynamics of cellular processes in living animals.34,35 Another exciting technical advance was the development and application of non-toxic, water soluble quantum dots (Qdots) to intravital imaging.36 For example, intravenous injection of Qdots enabled researchers to visualize microvasculature and the dynamics of blood flow in living tissues including the brain. Similarly, sulforhodamine 101 (SR101) was debuted around the same time as a red fluorescent dye that is specifically taken up by astrocytes and can be used to label these cells in the living brain.37,38 In addition to fluorescent markers, TPLSM researchers have also taken advantage of properties inherent to tissues of interest such as autofluorescence, second-harmonic generation and third-harmonic generation.39,40 Visualization of structures like collagen, retinol, NADPH, flavins, etc. can all be achieved by TPLSM without having to specifically label them.39 Collectively, the aforementioned tools represent just a few advances that have aided TPLSM researchers in their ability to study the living nervous system. With each passing year, the field further matures and continues to provide a plethora of new tools that make multidimensional, multi-color, high-resolution (spatial and temporal) TPLSM imaging in living tissues even easier to conduct. These advances propel researchers toward seminal discoveries in the fields of neuroscience and neuroimmunology, some of which will be highlighted in the paragraphs that follow.
Microglia
Our current understanding of microglial biology is deeply rooted in the seminal work of Pío del Río Hortega who first named these cells and is considered the “Father of Microglia.”41,42 Using a silver staining technique and a light microscope, he described the ramified morphology of microglia and predicted their phagocytotic capabilities. Subsequently, examination of autopsied brain tissue revealed that microglia become activated and transform into amoeboid cells during most CNS diseases that involve injury or perturbation of the parenchyma. Over the years, advancements made in microscopy, genetics, cell culture techniques, flow cytometry, and immunohistochemistry have enhanced our ability to probe microglial functions and have yielded significant breakthroughs. With current technology, it is now possible to capture microglial dynamics in the living nervous system as they respond to different challenges (Vid. S1).
Initial time-lapse imaging studies of microglia were conducted using a phase-contrast microscope to visualize these cells in cortical cell cultures. The data from these studies revealed that microglia extend and retract their processes, are sometimes motile, are morphologically plastic, and show a high degree pinocytotic activity.43,44 These seminal observations provided a foundation for microglia behavior that was later confirmed ex vivo and in vivo. Microglia dynamics were first studied ex vivo by Brockhaus and colleagues who used time lapse video microscopy to image the corpus callosum in brain slices.45 Microglia labeled with the fluorescent lipid, DiIC18, were shown to migrate to the cut surface of brain slices (which represents an injury response), actively survey the damaged area with their branched processes, and finally phagocytose dead cells. A few years later Dailey and Waite published another technique to label microglia with fluorescently-tagged plant isolectin (FITC-IB4).46 Using this approach, they imaged the endocytic and phagocytic properties of microglia in rat hippocampal brain slices with a scanning laser confocal microscope.46 In general, these microglial labeling approaches resulted in some background staining of other brain structures such as blood vessels; thus, the identification of microglia was often confirmed based on their unique morphological features. With further advances in labeling and 3D volumetric reconstructions, Stence et al. captured the temporal transformation of microglial activation as they changed morphologically from ramified to amoeboid in response to the acute mechanical injury that occurs when cutting brain slices.47 In concert, these studies demonstrated ex vivo that microglia are rapid responders to brain injury.
While ex vivo studies are insightful, all innate immune sentinels in the CNS (including microglia) are contextual cells whose behaviors are shaped by the niches in which they reside. Brain slices are commonly used to study the nervous system ex vivo, but it is important to note that the process of brain removal and cutting induces a rapid change (within minutes) in the dynamics and activation status of innate immune cells. Thus, it is not possible to study the normal physiology and function of these cells in brain slice cultures. To gain insights into microglia in vivo, researchers have opened windows into the brain by performing craniotomies or skull thinning, which allow visualization of the underlying brain parenchyma using imaging techniques such as TPLSM.16 Two landmark studies in 2005 revealed that microglia are stationary in the resting CNS, but their processes are highly dynamic and continually scan the parenchymal space around them.5,48 This observation was made by imaging the neocortex of living CX3CR1-GFP+/- mice6 with a two-photon microscope. Interestingly, these studies also showed that microglia rapidly respond to a laser-induced injury by extending their processes toward the focus of damage. Mechanistically, microglial process extension was mediated by ATP released from the damaged cells and surrounding astrocytes, which was detected by G protein-coupled purinergic receptors on the responding microglia.48 This pathway affords microglia and other innate immune sentinels with the ability to rapidly respond to cellular damage49—a response that declines with age.50 In fact, it is likely that microglia respond to similar signals generated along the cutting surface of ex vivo brain slices. Microglia are exquisitely sensitive to their surroundings and can flux calcium in response to the death of just a single neuron.51 More recently, it was revealed that even the type of cranial window used for intravital TPLSM studies can influence the behavior of microglia.52 Insertion of a glass window is typically preceded by a craniotomy during which a portion of the skull bone is removed. As expected, this results in disruption of the underlying meninges and induces a massive injury response that spreads across the ipsilateral hemisphere.52 To prevent this injury, a new approach was developed, which is referred to as skull thinning.52,53 This is achieved by gently thinning down the skull bone by hand. Importantly, use of a thinned skull window (if performed correctly) does not induce activation of brain resident innate immune sentinels, which allows them to be studied in a physiological setting. Ever since the first intra-vital TPLSM study of microglia was published,5 there has been great enthusiasm in the field. Our insights into microglia dynamics during states of health and disease has already improved and more pioneering studies are underway.
Microglia dynamics have been studied in several animal models of Alzheimer disease (AD). Progressive accumulation of β-amyloid (Aβ) deposits in extracellular spaces of the brain is a hallmark feature of AD. In most cases, these deposits (or plaques) are surrounded by activated microglia. To establish the role of microglia in AD, researchers have used several approaches including TPLSM. Using reporter mice that allow visualization of plaque formation, it was recently revealed that plaques can form quickly (within a day) and microglia become activated and recruited to the site shortly thereafter (1–2 d later).54 When microglia were depleted for a 4-week period using CD11b-thymidine kinase transgenic mice, no changes in plaque size or neuritic dystrophy were observed, suggesting that microglia do not influence amyloid deposition over this time interval.55 However, a role for microglia in AD pathogenesis was demonstrated in CX3CR1GFP/GFP mice (a knockout for CX3CR1) imaged by TPLSM.56 CX3CR1 is expressed by several CNS myeloid sentinels including microglia (Fig. 1; Vid. S1). Neurons express the ligand, CX3CL1, which can facilitate interactions with microglia. CX3CR1 deficiency did not alter the ability of microglia to phagocytose Aβ, but did significantly decrease neuronal cell death in AD mice.56 In contrast, another TPLSM study demonstrated that CX3CR1 deficiency increased the number and phagocytic ability of microglia around plaques in AD mice, but did not impact neuronal cell death.57 The discrepancy between these two studies might be explained by the different murine AD models that were used. At present, the exact role of CNS myeloid cells in the pathogenesis of AD is not entirely clear. Some studies suggest that parenchymal microglia are responsible for phagocytosing Aβ,56,58 whereas other have concluded that bone-marrow derived myeloid cells are more important.59 Either way, a therapeutic approach that should be considered in humans with AD is to improve Aβ uptake by both CNS-resident and peripherally-derived myeloid cells.
Microglial dynamics have also been examined during vascular events that occur in the CNS. For example, Rosidi and colleagues recently studied the innate myeloid response to a cortical microhemorrhage induced by a focal laser injury.60 As expected, microglia responded within hours by extending processes directionally toward the damaged blood vessel, and the distance of responding cells in relation to the lesion was relatively short (~90 μm from the injury). Following an ischemic event, regional blood flow can influence microglia dynamics.61 Interestingly, it was also demonstrated in a recent study that microglia interact directly with neuronal synapses for up to an hour following a transient ischemic event.62 Under steady-state conditions, microglia make brief contacts with synapses that last ~5 min; however, following an ischemic event the duration of these interactions increases significantly. It is postulated that these post-ischemic interactions contribute to synaptic remodeling, as they were often followed by disappearance of the presynaptic bouton. This theory is consistent with the general role that microglia play in synaptic remodeling in the healthy brain.63
Because our understanding of microglia cannot rely on invasive imaging techniques alone, it is important to develop and employ noninvasive imaging strategies that are ultimately useful in human subjects. Imaging of single iron labeled cells by MRI is already possible with a spatial resolution of ~50 μm.64 With an increase in temporal resolution, it should soon be possible to obtain dynamic information about iron-labeled cells of interest. The application non-invasive imaging modalities to humans are particularly important for the assessment of cellular activation. Microglia and other brain-resident innate immune cells are remarkably sensitive to local perturbations and can serve as barometers for CNS diseases. Thus, it is incredibly important to develop non-invasive imaging techniques to assess the activation status of innate immune sentinels in the CNS. For example, a PET based imaging approach involving a radio-labeled tracers was recently used to detect activated microglia in the thalamus of patients with ischemic stroke and dementia.65-67 This technique exploits the fact that microglia increase expression of the benzodiazepine binding receptor upon activation, and the PET tracer, carbon 11-labeled (R)-PK11195, has high a specificity for this receptor. The presence of activated microglia around amyloid plaques was also successfully mapped by PET in the cortex of human subjects with Alzheimer disease.68 These studies demonstrate the feasibility of using non-invasive imaging techniques like PET to probe the activation status of innate immune sentinels in the nervous system of humans.
Juxtavascular Microglia
As their name suggests, juxtavascular microglia are anatomically positioned adjacent to parenchymal blood vessels and are in direct contact with the glial limitans.69-71 Because of their close proximity to the blood brain barrier (BBB), juxtavascular microglia are thought to respond to vascular signals and perhaps even sample blood components at the perivascular glia limitans;72 however, there is little information about the cellular dynamics of these cells in vivo. One study by Grossmann et al. used time-lapse imaging of rat hippocampal brain slices to study the dynamics of FITC-IB4 stained juxtavascular microglia in response to the cutting injury.70 This study concluded that juxtavascular microglia respond more quickly to injury than microglia not associated with blood vessels. In addition, juxtavascular microglia were observed moving along the brain microvessels in response to traumatic injury. It remains to be determined whether these cells respond similarly in vivo given that the generation of brain slices results in a complete disconnection from the vascular system, which has the potential to trigger atypical cellular behaviors. Thus, it will be important in future studies to monitor juxtavascular microglia intravitally through a thinned skull window and determine whether these cells do indeed sample vascular components and respond to injury by migrating along parenchymal vessels. It will also be important to develop markers to distinguish juxtavascular microglia from perivascular macrophages (a bone-marrow derived cell).
Perivascular Macrophages
Perivascular macrophages reside in the perivascular spaces around subpial blood vessels that enter the brain (referred to as the Virchow-Robin space) as well as parenchymal blood vessels. These cells are not ramified like microglia, but instead have flat, slightly elongated cell bodies that allow them to wrap around vessel walls and scan the contents of the perivascular space (Fig. 1E and 2E; Vids. S1 and S2).73 Hickey and Kimura were the first to demonstrate that perivascular macrophages are bone marrow-derived APCs capable of presenting antigen to lymphocytes.74 Additional studies revealed that perivascular macrophages are replenished by blood-derived myeloid cells and that this occurs as early as two weeks post-bone marrow transplantation.75 It was similarly demonstrated in a murine model of amyotrophic lateral sclerosis (ALS) that CX3CR1-GFP+/- myeloid cells from the bone marrow entered the CNS and inhabited the perivascular spaces as disease developed.76 Collectively, these data indicate that bone marrow-derived myeloid cells can cross the BBB and differentiate into perivascular macrophages during physiological and pathophysiological conditions.
Perivascular macrophages respond quickly to systemic immune challenges as evidenced by upregulation of cyclooxygenenase-2 following peripheral administration of lipolysaccharide (LPS).77 Peripheral LPS injection also induces IL-1β production in cells adjacent to parenchymal blood vessels, which include perivascular macrophages.78 Following disruption of vascular barriers, perivascular macrophages play an important role in scavenging materials that traverse endothelial tight junctions. For example, intravenously injected horseradish peroxidase, low density lipoprotein, and ferritin accumulate in perivascular phagocytes following barrier activation with IL-1β or TNF-α.79,80 The scavenging capacity of perivascular macrophages is further supported by studies showing that these cells express mannose receptors that facilitate the uptake of complex carbohydrates.81,82 Because of their anatomical position and ability to rapidly respond to blood-derived stimuli, perivascular macrophages should be considered among the most important innate immune sentinels in the CNS. These cells provide an early warning system to the brain parenchyma about peripheral challenges that have gained access to the blood supply.
Following activation, perivascular macrophages initiate rapid defense mechanisms to protect the brain. Given their position along blood vessels, these cells are considered potent modulators of CNS immune activity and are critical for protection against invading microbes.83,84 For example, protection against cryptococcus neoformans, a fungus that causes meningitis and encephalitis in immunosuppressed humans, is mediated by CD4+ T cells and MHC II expression on perivascular macrophages (not parenchymal microglia).84 Perivascular macrophages also serve as a reservoir for human immunodeficiency virus (HIV) in humans, even before neurological symptoms develop.85 Infection of these bone marrow-derived myeloid cells likely provides HIV with a gateway into the CNS. In addition, activation of perivascular macrophages and other CNS myeloid sentinels by HIV has the potential to trigger neurological dysfunction.86 At present, perivascular macrophages are thought to be important in many CNS inflammatory conditions. Because many perivascular myeloid cells also express DC markers (e.g., CD11c),13 it will be important to define the exact lineage of all CNS perivascular immune cells as well as how they directly contribute to various inflammatory conditions. This opportunity is now available given our ability to visualize perivascular myeloid cells by TPLSM using fluorescent protein reporter mice (Figs. 1 and 2; Vids. S1 and S2).
Dendritic Cells
Consistent with its immunoprivileged status,87 the CNS was once considered a tissue without DCs. Intuitively, this made sense because DCs are potent APCs, and the CNS is intolerant of fulminant inflammatory responses. However, upon further investigation, it became clear that the CNS maintains a population of CD11c+ DC-like cells that bear resemblance to splenic DCs.9,10,12,15 These cells are bone marrow-derived and reside primarily in the meninges, choroid plexus, perivascular spaces, and juxtavascular parenchyma.12,13 They also have the ability to present self-antigen and stimulate T cells.10 As mentioned, CD11c-YFP reporter mice11 are commonly used to study CNS resident DCs;9,10,12,15 however, many different cell lineages can express CD11c, particularly after an inflammatory challenge. Microglia, meningeal macrophages, perivascular macrophages, etc. all have the capacity to express CD11c, which must be considered when non-specifically classifying all CD11c+ cells as DCs. Under steady-state conditions, TPLSM time-lapses have revealed that CD11c-YFP expressing cells in the meninges are morphologically distinct from worm-like meningeal macrophages, but some do look similar to perivascular macrophages visible in CX3CR1-GFP and LysM-GFP reporter mice (Fig. 3; Vid. S3). CNS DCs are of a myeloid lineage based on their CD11b expression8,13,15 and resemble in many ways the bone-marrow derived perivascular APCs that Hickey and Kumura described in 1988.74 While there has been some confusion with nomenclature over the years, it is clear that bone marrow-derived, CNS-resident CD11c+ cells are excellent antigen presenters.8,9,15,88
With functional capabilities on par with their peripheral counterparts, CNS DCs actively participate in most inflammatory processes within the nervous system.15 For example, following viral8,9 and parasitic89 infections, CD11c+ cells accumulate in the brain. This increased number of CD11c+ cells is explained by upregulation of CD11c on activated microglia as well as infiltration of blood-derived DCs.9 A recent TPLSM study of Toxoplasma gondii immunity in acute brain slices revealed that CD11c-YFP cells change in morphology from amoeboid (resting) to dendriform (activated) following infection and interact directly with parasite-specific CD8+ T cells.89 In these studies, the T cells were highly motile, whereas the CD11c-YFP+ APCs were sessile. CD11c-YFP+ APCs were also shown to influence the cell cycle program of virus-specific CD8+ T cells by promoting stable arrest and mitosis during viral meningitis.90 Cytotoxic lymphocytes (CTL) can migrate through the blood in active stages of cell cycle and then complete this cell cycle program by splitting into daughter cells (cytokinesis) upon interaction with cognate peptide-MHC I bearing APCs residing in the virally infected meninges.90 Collectively, these studies demonstrate that CD11c+ APCs can promote T cell division and modulate their effector functions in the infected CNS.
CD11c-expressing cells have also been observed in the CNS during other inflammatory conditions such as ischemia and autoimmunity.15,91-93 Following cerebral ischemia, brain resident CD11c+ cells were found at the infarct border, whereas peripherally-derived CD11c+ cells populated the lesion core.91 These cells had increased expression of MHC II and co-stimulatory molecules, but how they contribute to infarct development or resolution remains unknown. The importance of CD11c+ cells in the development of experimental autoimmune encephalomyelitis (EAE), a CNS autoimmune disease, is better understood. Induction of EAE involves immunization with myelin components or adoptive transfer of myelin-specific CD4+ T cells. Disease development is critically dependent on interactions with CNS APCs,92-94 and recent studies showed that bone marrow-derived perivascular CD11c+ cells alone can stimulate myelin-reactive CD4+ T cells and promote disease development.92 This finding is supported by a seminal TPLSM study in which myelin-specific and bystander CD4+ T cells were visualized in the spinal cord of living animals during the development of EAE.27 Both myelin-specific and bystander T cells were capable of extravasating from blood vessels and migrating through the meninges and perivascular spaces; however, only myelin-reactive cells could enter the spinal cord parenchyma and this occurred after engagement of antigen-bearing local APCs. It is presently unclear which CNS APC(s) can support T cell movement from the meninges into the parenchyma, but it is assumed that bone marrow-derived CD11c+ APCs play a critical role in this process.
Meningeal and Choroid Plexus Macrophages
Meningeal and choroid plexus macrophages are bone-marrow derived myeloid cells that reside in the anatomical niches denoted by their names. Because the barrier structures in the meninges and choroid plexus are not as secure as the BBB in the CNS parenchyma, it is important to have a vigilant sentinel program in these anatomical compartments. At present, there is almost no dynamic information about these specialized macrophages. Imaging choroid plexus macrophages would pose a particular challenge given their deep localization in the ventricular system. Meningeal macrophages, on the other hand, are more accessible although still understudied. The worm-like macrophages visible in LysM-GFP mice line most meningeal blood vessels (Figs. 1C and 2C; Vids. S1 and S2) and thus could be considered a perivascular macrophage of the meninges. Interestingly, there are other morphologies visible in the meninges of LysM-GFP mice (Fig. 2E; Vid. S2), and it is presently unclear whether these cells are CD11c+ DCs or another specialized lineage altogether. Overall, there is still much to be learned about the innate immune cells that inhabit the meninges and ventricular system.
Given their proximity to the blood supply and less fortified barriers, it is predicted that the meninges and choroid plexus would serve as portals for leukocyte entry into the CNS. This notion is supported by a recent study demonstrating that CCR6-deficient mice are resistant to EAE.95 Interestingly, CCL20, the ligand for CCR6, is expressed on epithelial cells that comprise the choroid plexus. In the absence of CCR6, myelin-reactive CD4+ T cells became trapped in the choroid plexus and could not enter the CNS parenchyma to cause disease. These data combined with the recent TPLSM work by Bartholomaus and colleagues27 demonstrate that the choroid plexus and meninges provide portals into the CNS, and the gatekeepers are anatomically specialized macrophages and dendritic cells that are continually replenished by the bone marrow.
Monocytes
Under steady-state conditions, the CNS is surveyed by a small pool of resting monocytes (CD62L+Ly6ClowCX3CR1high), and their numbers increase dramatically during acute and chronic inflammation (CD62L+Ly6ChiCX3CR1low), mostly due to infiltration from the periphery.96,97 Because monocytes are myeloid cells, they often give rise to tissue-specific macrophages during inflammation. Infiltration of circulating Ly6C+ monocytes into the CNS and their subsequent participation in CNS pathogenesis is well documented during states of injury, infection, and autoimmunity.26,96,98-100 In fact, it was reported recently that Ly6ChiCCR2+ circulating monocytes can migrate into the CNS parenchyma and convert into microglia.101,102 However, this conversion was dependent on first pre-conditioning the brain with irradiation. Using parabiotic (non-irradiated) animals, it was later demonstrated that monocytes do not in fact convert into microglia under physiological or pathophysiological conditions.100 Inhibition of monocyte recruitment into the CNS revealed that they are required for the development of EAE; however, they do not remain in the parenchyma as microglia after the disease subsides.100
We are just beginning to gain insights into the dynamics of monocytic surveillance of the CNS during steady-state and inflammatory conditions. Auffray and colleagues were the first to observe in the periphery that CX3CR1-GFP+ expressing monocytes routinely survey uninflamed blood vessels by crawling along endothelium in a LFA-1 dependent manner.103 This patrolling behavior was required for rapid tissue entry following infection with Listeria monocytogenes. Vascular patrolling by monocytes has also been observed in CNS vessels.104 Following direct injection of GFP-labeled monocytes into an entorhinal cortex lesion, some cells migrated out of the CNS into the deep cervical lymph nodes (CLN).99 The cells traveled along the olfactory nerves in transit to the CLN. These data suggest that monocytes can exit the brain, although the importance of this finding remains unclear. During viral meningitis, a recent TPLSM study demonstrated that myelomonocytic cells (which include monocytes and neutrophils) can contribute to severe vascular damage.26 In LysM-GFP reporter mice infected with LCMV, imaging through a thinned skull window revealed that neutrophils damage meningeal blood vessels by synchronous extravasation, whereas monocyte-derived cells localized perivascularly and appeared to damage blood vessels using a different (currently unknown) mechanism. Importantly, these cells were recruited to the meninges in part by chemokines released by infiltrating CTL. Given the contribution of monocytes to the pathogenesis of many different CNS inflammatory conditions, it is clear that while these cells are essential for a potent anti-microbial defense, they can also contribute to severe vascular and parenchymal pathology. Thus, it will be important to gain additional insights into the in vivo dynamics of these cells as they participate in pathogenic vs. non-pathogenic activities within the CNS.
Future Perspectives
The CNS like most tissues is inhabited by an elaborate network of innate immune sentinels that bear some specialized functions based on the anatomical niche in which they reside. Over the years we have learned a great deal about these CNS residents, especially how they contribute to various inflammatory processes. These cells are poised to protect and even repair the CNS when needed. The recent application of intravital TPLSM to the study of CNS sentinels has markedly improved our understanding of how these cells function in the living brain (particularly if imaged through a thinned skull window). The advantage of using this approach is that immune sentinels can be studied in physiological micro-environments, which is important because these cells are heavily influenced by their surroundings. Extraction from the CNS environment changes their morphology and physiology. Because of their overall abundance and accessibility, microglia are the most commonly studied innate immune cells in the CNS by TPLSM. Much less is known about the dynamics of meningeal, choroid plexus, and perivascular sentinels. Using modern tools, it is now possible to monitor all of these cells in the living CNS during states of health and disease. The advantage of TPLSM is that labeled cells of interest can be studied anatomically and temporally in their native environment. This is particularly important for delicate cells or those with long processes (e.g., microglia and dendritic cells) that become damaged upon extraction. With TPLSM, the transformation of an innate cell from a resting to an activated state can be visualized in real-time, thus eliminating questions about lineage derivation. In general, there is still some confusion about innate immune cell lineages in the CNS and how they uniquely contribute to inflammatory processes. Microglia, macrophages, and dendritic cells can all share common markers (e.g., CD11c) and are sometimes lumped together when studying CNS inflammatory responses. Using morphological features in combination with different reporter mice (e.g., Figs. 1-3; Vids. S1-S3), it should be possible to define anatomical niche-specific immune responses in their entirety. Given the technologies in hand, we have arrived at an exciting time in the field of neurobiology when neural-immune interactions can be studied in living systems in real-time.
Supplementary Material
Footnotes
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/intravital/article/22823
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–62. doi: 10.1038/nature09615. http://dx.doi.org/10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 2.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–35. doi: 10.1038/nn.2923. http://dx.doi.org/10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. http://dx.doi.org/10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. http://dx.doi.org/10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. http://dx.doi.org/10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 6.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. http://dx.doi.org/10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–26. [PubMed] [Google Scholar]
- 8.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203:1963–75. doi: 10.1084/jem.20060039. http://dx.doi.org/10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Agostino PM, Kwak C, Vecchiarelli HA, Toth JG, Miller JM, Masheeb Z, et al. Viral-induced encephalitis initiates distinct and functional CD103+ CD11b+ brain dendritic cell populations within the olfactory bulb. Proc Natl Acad Sci U S A. 2012;109:6175–80. doi: 10.1073/pnas.1203941109. http://dx.doi.org/10.1073/pnas.1203941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–705. doi: 10.1084/jem.20102657. http://dx.doi.org/10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. http://dx.doi.org/10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 12.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. http://dx.doi.org/10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 13.Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–58. doi: 10.1007/s00401-010-0774-y. http://dx.doi.org/10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 14.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98:6295–300. doi: 10.1073/pnas.111152498. http://dx.doi.org/10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: biology and pathology. Acta Neuropathol. 2012;124:599–614. doi: 10.1007/s00401-012-1018-0. http://dx.doi.org/10.1007/s00401-012-1018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–29. doi: 10.1038/nri2971. http://dx.doi.org/10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herz J, Zinselmeyer BH, McGavern DB. Two-photon imaging of microbial immunity in living tissues. Microsc Microanal. 2012;18:730–41. doi: 10.1017/S1431927612000281. http://dx.doi.org/10.1017/S1431927612000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–6. doi: 10.1126/science.2321027. http://dx.doi.org/10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 19.Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–4. doi: 10.1038/375682a0. http://dx.doi.org/10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 20.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A. 1998;95:15741–6. doi: 10.1073/pnas.95.26.15741. http://dx.doi.org/10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller J, Schiller Y, Clapham DE. NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation. Nat Neurosci. 1998;1:114–8. doi: 10.1038/363. http://dx.doi.org/10.1038/363. [DOI] [PubMed] [Google Scholar]
- 22.Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–81. doi: 10.1038/35009107. http://dx.doi.org/10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 23.Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–6. doi: 10.1073/pnas.2133652100. http://dx.doi.org/10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. http://dx.doi.org/10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 25.Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, et al. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. http://dx.doi.org/10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–5. doi: 10.1038/nature07591. http://dx.doi.org/10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–8. doi: 10.1038/nature08478. http://dx.doi.org/10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 28.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–9. doi: 10.1038/nature11337. http://dx.doi.org/10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 29.Denk W, Sugimori M, Llinás R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1995;92:8279–82. doi: 10.1073/pnas.92.18.8279. http://dx.doi.org/10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denk W, Holt JR, Shepherd GMG, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–21. doi: 10.1016/0896-6273(95)90010-1. http://dx.doi.org/10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 31.Potter SM, Wang C-M, Garrity PA, Fraser SE. Intravital imaging of green fluorescent protein using two-photon laser-scanning microscopy. Gene. 1996;173(1 Spec No):25–31. doi: 10.1016/0378-1119(95)00681-8. http://dx.doi.org/10.1016/0378-1119(95)00681-8. [DOI] [PubMed] [Google Scholar]
- 32.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–9. doi: 10.1038/nmeth819. http://dx.doi.org/10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 33.Mainen ZF, Maletic-Savatic M, Shi SH, Hayashi Y, Malinow R, Svoboda K. Two-photon imaging in living brain slices. Methods. 1999;18:231–9. 181. doi: 10.1006/meth.1999.0776. http://dx.doi.org/10.1006/meth.1999.0776. [DOI] [PubMed] [Google Scholar]
- 34.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A. 1998;95:15741–6. doi: 10.1073/pnas.95.26.15741. http://dx.doi.org/10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–5. doi: 10.1038/385161a0. http://dx.doi.org/10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 36.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–6. doi: 10.1126/science.1083780. http://dx.doi.org/10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 37.Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–7. doi: 10.1038/nmeth706. http://dx.doi.org/10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 38.Appaix F, Girod S, Boisseau S, Römer J, Vial JC, Albrieux M, et al. Specific in vivo staining of astrocytes in the whole brain after intravenous injection of sulforhodamine dyes. PLoS One. 2012;7:e35169. doi: 10.1371/journal.pone.0035169. http://dx.doi.org/10.1371/journal.pone.0035169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100:7075–80. doi: 10.1073/pnas.0832308100. http://dx.doi.org/10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witte S, Negrean A, Lodder JC, de Kock CP, TestaSilva G, Mansvelder HD, et al. Label-free live brain imaging and targeted patching with third-harmonic generation microscopy. Proc Natl Acad Sci U S A. 2011;108:5970–5. doi: 10.1073/pnas.1018743108. http://dx.doi.org/10.1073/pnas.1018743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Rio-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. New York: P.B. Hoeber, inc; 1937. pp. 481–534. [Google Scholar]
- 42.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. http://dx.doi.org/10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 43.Booth PL, Thomas WE. Evidence for motility and pinocytosis in ramified microglia in tissue culture. Brain Res. 1991;548:163–71. doi: 10.1016/0006-8993(91)91118-k. http://dx.doi.org/10.1016/0006-8993(91)91118-K. [DOI] [PubMed] [Google Scholar]
- 44.Ward SA, Ransom PA, Booth PL, Thomas WE. Characterization of ramified microglia in tissue culture: pinocytosis and motility. J Neurosci Res. 1991;29:13–28. doi: 10.1002/jnr.490290103. http://dx.doi.org/10.1002/jnr.490290103. [DOI] [PubMed] [Google Scholar]
- 45.Brockhaus J, Möller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16:81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. http://dx.doi.org/10.1002/(SICI)1098-1136(199601)16:1<81∷AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–30. 177. doi: 10.1006/meth.1999.0775. http://dx.doi.org/10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- 47.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–66. http://dx.doi.org/10.1002/1098-1136(200103)33:3<256∷AID-GLIA1024>3.0.CO;2-J. [PubMed] [Google Scholar]
- 48.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. http://dx.doi.org/10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 49.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–12. doi: 10.1038/nri2938. http://dx.doi.org/10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–76. doi: 10.1111/j.1474-9726.2010.00660.x. http://dx.doi.org/10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eichhoff G, Brawek B, Garaschuk O. Microglial calcium signal acts as a rapid sensor of single neuron damage in vivo. Biochim Biophys Acta. 2011;1813:1014–24. doi: 10.1016/j.bbamcr.2010.10.018. http://dx.doi.org/10.1016/j.bbamcr.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–51. doi: 10.1038/nn1883. http://dx.doi.org/10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 53.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–8. doi: 10.1038/nprot.2009.222. http://dx.doi.org/10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–4. doi: 10.1038/nature06616. http://dx.doi.org/10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grathwohl SA, Kälin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, et al. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–3. doi: 10.1038/nn.2432. http://dx.doi.org/10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuhrmann M, Bittner T, Jung CKE, Burgold S, Page RM, Mitteregger G, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010;13:411–3. doi: 10.1038/nn.2511. http://dx.doi.org/10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30:17091–101. doi: 10.1523/JNEUROSCI.4403-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–63. doi: 10.1073/pnas.0909586107. http://dx.doi.org/10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simard AR, Soulet D, Gowing G, Julien J-P, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. http://dx.doi.org/10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 60.Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, et al. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6:e26612. doi: 10.1371/journal.pone.0026612. http://dx.doi.org/10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–53. doi: 10.1002/glia.21220. http://dx.doi.org/10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. http://dx.doi.org/10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. http://dx.doi.org/10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9. doi: 10.1002/mrm.20718. http://dx.doi.org/10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 65.Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–4. doi: 10.1212/wnl.55.7.1052. http://dx.doi.org/10.1212/WNL.55.7.1052. [DOI] [PubMed] [Google Scholar]
- 66.Gerhard A, Neumaier B, Elitok E, Glatting G, Ries V, Tomczak R, et al. In vivo imaging of activated microglia using [11C]PK11195 and positron emission tomography in patients after ischemic stroke. Neuroreport. 2000;11:2957–60. doi: 10.1097/00001756-200009110-00025. http://dx.doi.org/10.1097/00001756-200009110-00025. [DOI] [PubMed] [Google Scholar]
- 67.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–7. doi: 10.1016/S0140-6736(01)05625-2. http://dx.doi.org/10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 68.Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R) PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–9. doi: 10.1016/j.nbd.2008.08.001. http://dx.doi.org/10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–87. doi: 10.1016/0165-0173(94)00015-h. http://dx.doi.org/10.1016/0165-0173(94)00015-H. [DOI] [PubMed] [Google Scholar]
- 70.Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–40. http://dx.doi.org/10.1002/glia.10031. [PubMed] [Google Scholar]
- 71.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103. doi: 10.1002/glia.20990. http://dx.doi.org/10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 72.Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–43. doi: 10.1002/jnr.490280211. http://dx.doi.org/10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- 73.Mander TH, Morris JF. Immunophenotypic evidence for distinct populations of microglia in the rat hypothalamo-neurohypophysial system. Cell Tissue Res. 1995;280:665–73. doi: 10.1007/BF00318369. http://dx.doi.org/10.1007/BF00318369. [DOI] [PubMed] [Google Scholar]
- 74.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. http://dx.doi.org/10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 75.Bechmann I, Priller J, Kovac A, Böntert M, Wehner T, Klett FF, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–8. doi: 10.1046/j.0953-816x.2001.01793.x. http://dx.doi.org/10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 76.Lewis CA, Solomon JN, Rossi FM, Krieger C. Bone marrow-derived cells in the central nervous system of a mouse model of amyotrophic lateral sclerosis are associated with blood vessels and express CX(3) CR1. Glia. 2009;57:1410–9. doi: 10.1002/glia.20859. http://dx.doi.org/10.1002/glia.20859. [DOI] [PubMed] [Google Scholar]
- 77.Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–29. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. http://dx.doi.org/10.1002/(SICI)1096-9861(19970505)381:2<119∷AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 78.Van Dam AM, Bauer J, Tilders FJH, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 β in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–26. doi: 10.1016/0306-4522(94)00549-k. http://dx.doi.org/10.1016/0306-4522(94)00549-K. [DOI] [PubMed] [Google Scholar]
- 79.Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci U S A. 1996;93:3269–74. doi: 10.1073/pnas.93.8.3269. http://dx.doi.org/10.1073/pnas.93.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claudio L, Martiney JA, Brosnan CF. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab Invest. 1994;70:850–61. [PubMed] [Google Scholar]
- 81.Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–72. doi: 10.1084/jem.189.12.1961. http://dx.doi.org/10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH, Boche D. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–84. doi: 10.1002/glia.20124. http://dx.doi.org/10.1002/glia.20124. [DOI] [PubMed] [Google Scholar]
- 83.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–64. doi: 10.1002/glia.1105. http://dx.doi.org/10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 84.Aguirre K, Miller S. MHC class II-positive perivascular microglial cells mediate resistance to Cryptococcus neoformans brain infection. Glia. 2002;39:184–8. doi: 10.1002/glia.10093. http://dx.doi.org/10.1002/glia.10093. [DOI] [PubMed] [Google Scholar]
- 85.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–9. doi: 10.1016/j.ajpath.2011.06.039. http://dx.doi.org/10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–62. doi: 10.1146/annurev.neuro.25.112701.142822. http://dx.doi.org/10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 87.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. http://dx.doi.org/10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci U S A. 2009;106:20918–23. doi: 10.1073/pnas.0911509106. http://dx.doi.org/10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, et al. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7:e1002246. doi: 10.1371/journal.ppat.1002246. http://dx.doi.org/10.1371/journal.ppat.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang SS, Herz J, Kim JV, Nayak D, Stewart-Hutchinson P, Dustin ML, et al. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J Exp Med. 2011;208:747–59. doi: 10.1084/jem.20101295. http://dx.doi.org/10.1084/jem.20101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24:724–37. doi: 10.1016/j.bbi.2009.11.002. http://dx.doi.org/10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34. doi: 10.1038/nm1197. http://dx.doi.org/10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 93.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–9. doi: 10.1038/nm1202. http://dx.doi.org/10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 94.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–52. doi: 10.1038/nm1177. http://dx.doi.org/10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 95.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–23. doi: 10.1038/ni.1716. http://dx.doi.org/10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 96.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–7. doi: 10.1182/blood-2008-07-168575. http://dx.doi.org/10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. http://dx.doi.org/10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 98.Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–500. doi: 10.1093/brain/awp144. http://dx.doi.org/10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 99.Kaminski M, Bechmann I, Pohland M, Kiwit J, Nitsch R, Glumm J. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leuko Biol. 2012 doi: 10.1189/jlb.0511241. [DOI] [PubMed] [Google Scholar]
- 100.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FMV. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–9. doi: 10.1038/nn.2887. http://dx.doi.org/10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 101.Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61. doi: 10.1038/nm1201-1356. http://dx.doi.org/10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 102.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch U-K, Mack M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. http://dx.doi.org/10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 103.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. doi: 10.1126/science.1142883. http://dx.doi.org/10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 104.Audoy-Rémus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallières L. Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci. 2008;28:10187–99. doi: 10.1523/JNEUROSCI.3510-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


