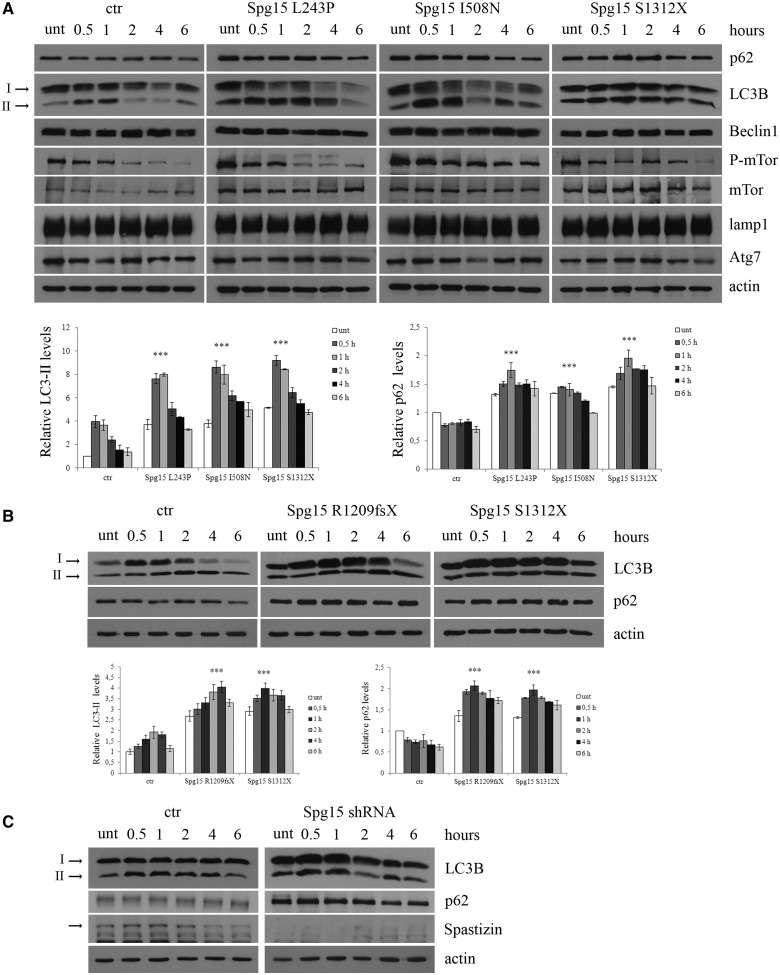
Figure 8.
Spastizin mutations induce an increase in LC3-II and p62 levels. (A) Lymphoblastoid cells from patients carrying L243P, I508N and S1312X spastizin mutations, three healthy control individuals and one healthy carrier from the I508N mutated family were starved with Earle’s Balanced Salt Solution for different times, from 30 min to 6 h to induce autophagy. Untreated and treated cells were lysed and total protein extracts were run on 6 or 10% SDS-polyacrylamide gels and probed with anti-p62, LC3B, -Beclin 1, -mTor, -phospho-mTor (P-mTor), -LAMP1, -Atg7 and -actin antibodies. In the figure is shown only one control (ctr), whereas results from the remaining three controls are reported in Supplementary Fig. 7A. Arrows indicate the two isoforms LC3-I and LC3-II. LC3-II and p62 levels were quantified and normalized on actin levels. The graphs show the mean ± SEM of three independent experiments (***P < 0.001). The mean of all the four controls is reported in the graphs. (B) Fibroblast cells from patients carrying R1209fsX and S1312X spastizin mutations and from a control were starved as in A to induce autophagy. Total protein extracts were run on 12% SDS-polyacrylamide gels and probed with anti-LC3B, -p62 and -actin antibodies. Shown is a representative of three reproducible blots. LC3-II and p62 levels were quantified and normalized on actin levels. The graphs show the mean ± SEM of three independent experiments (***P < 0.001). (C) Silencing of ZFYVE26 increases LC3-II and p62 levels. HeLa cells were transfected with Spg15 short hairpin RNA or with a control vector (ctr) and 72 h later were starved to induce autophagy. Total extracts were probed with anti-LC3B, -p62, -actin and -spastizin antibodies, the latter to show the residual spastizin levels in ZFYVE26 (Spg15) short hairpin RNA-transfected cells. Shown is a representative of three reproducible blots.