Fig. 6. In vivo.
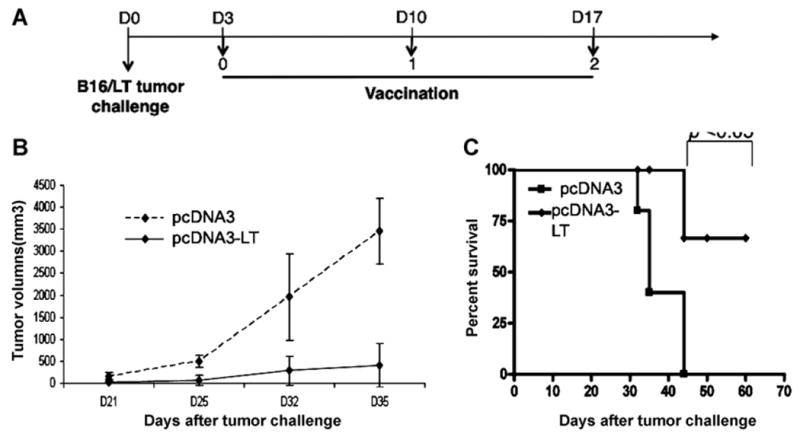
tumor treatment experiments. (A) Schematic diagram of the treatment regimen with either pcDNA3-LT DNA vaccine or control pcDNA3 empty vector. C57BL/6 mice (5 per group) were subcutaneously inoculated with 1 × 105 B16/LT tumor cells per mouse in the right flank on Day 0 (D0). The B16/LT-tumor bearing mice were treated with pcDNA3-LT or pcDNA3 intramuscularly followed by electroporation three times at a 1-week interval beginning on Day 3. Mice were monitored for evidence of tumor growth by inspection and palpation. Tumor growth was measured twice a week starting on Day 8. (B) Line graph depicting the tumor volume in B16/LT tumor-bearing mice treated with pcDNA3 or pcDNA3-LT DNA vaccine. (C) Kaplan–Meier survival analysis of B16/LT tumor-bearing mice treated with pcDNA3 or pcDNA3-LT DNA vaccine. Data shown are representative of two experiments performed.
