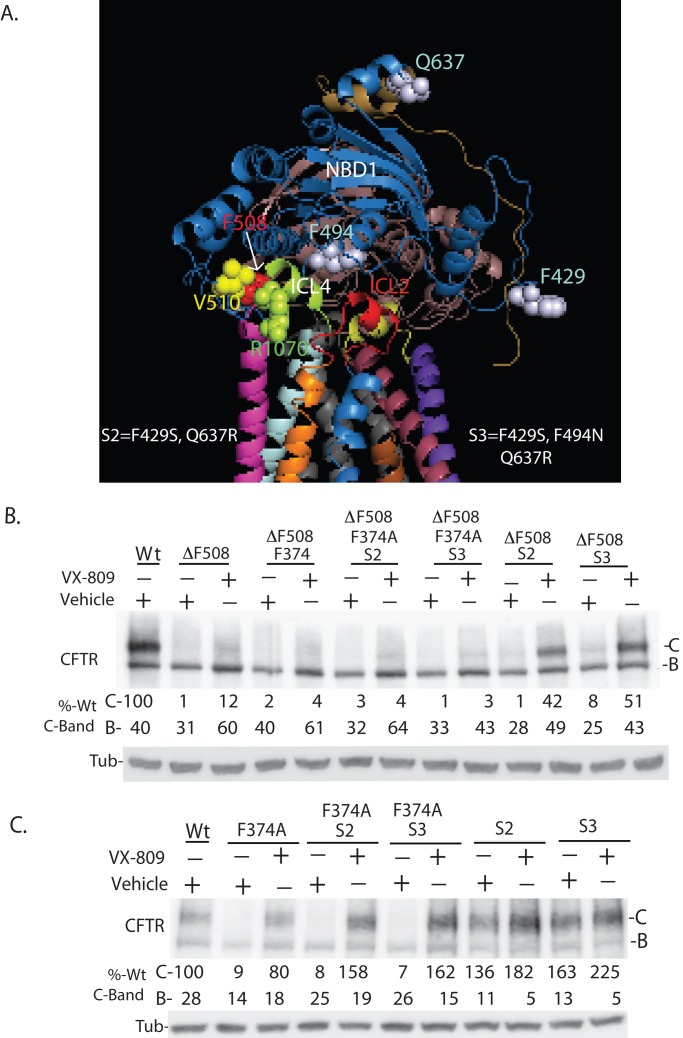
FIGURE 5:
Interdomain communication between MSD1 and NBD1 is required for VX-809 to enhance biosynthetic processing of CFTR. (A) Schematic of CFTR showing the position of indicated amino acid residues and domains. (B) Mutation of F374A hinders the ability of misfolding suppressor mutations S2 and S3 to increase the efficacy of VX-809 on F508del-CFTR. (C) Mutation of F374A hinders the ability of S2 and S3 to increase accumulation of the folded C-band of CFTR. Data in panels B and C are from Western blots of cell extracts. S2 (F429S, Q637R) and S3 (F429S, F494N, and Q637R) are mutations introduced into NBD1 to increase the thermodynamic stability of NBD1 and thereby increase CFTR and F508del-CFTR (B and C) folding efficiency (Pissarra et al., 2008; Teem et al., 1993). Panel are representations of three experiments. Quantitation is normalized to 100% of total C-band for CFTR detected for wild type. Bands B and C denote the ER-localized and plasma membrane–localized forms of CFTR.