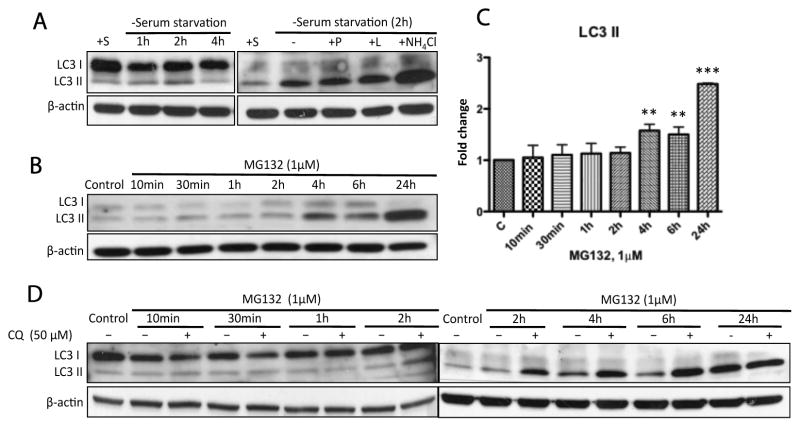
Figure 4. Prolonged proteasomal inhibition by MG132 stimulated time-dependent autophagic flux.
(A) N27 cells were confirmed to be autophagy-competent. LC3 II accumulation is a marker for autophagosome formation. (B) The representative immunoblot shows autophagy induction following treatment with MG132 (1μM). (C) Significant accumulation of LC3 II could be detected at 4h and to a greater extent at 24h. Fold changes of LC3 II levels are normalized by control and estimated by densitometry. These data are presented as mean ± SD, (n=3); **p<0.01 and ***p<0.001 are considered significant by ANOVA using Dunnet’s multiple comparison test. (D) Induction of autophagy by MG132 was confirmed by co-incubation with chloroquine (CQ). Cells were exposed to MG132 (1μM) with or without the co-treatment of CQ for the indicated times and then processed for immunoblot analysis. Note that CQ is a specific lysosome inhibitor and could suppress the fusion of autophagosomes with lysosomes. The elevated levels of LC3 II in the presence of CQ (>4h) excluded the possibility that MG132 stimulated autophagic flux was due to the blockade of autophagic degradation in N27 cells.