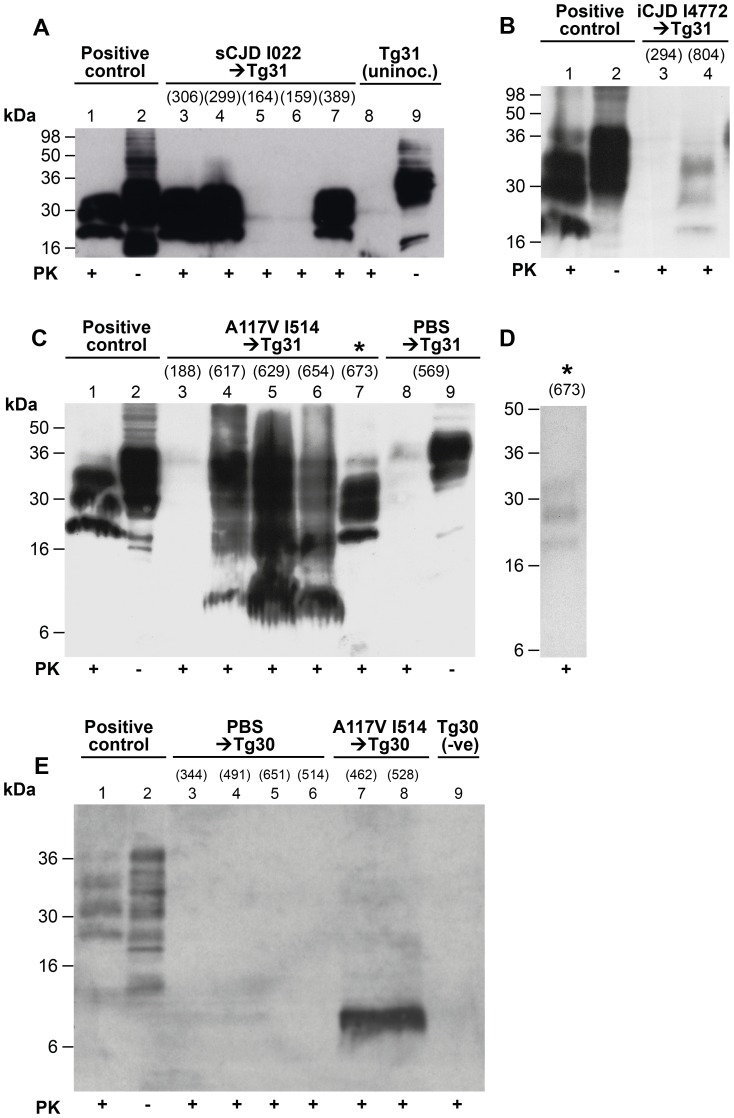
Figure 3. Immunoblot analysis of abnormal PrP propagated in the brains of 117VV transgenic mice challenged with IPD A117V and classical CJD.
Mice were inoculated with classical CJD and GSS A117V brain. Immunoblots were analysed by enhanced chemiluminescence with monoclonal anti-PrP antibody ICSM 35. The numbers in parentheses above relevant lanes, represent the number of days each mouse survived post-inoculation. The provenance of each brain sample is designated above each lane. (A) Immunoblots of sporadic CJD-inoculated 117VV Tg31 mice (lanes 3–7) showing PrPSc resistant to harsh proteinase K (PK) digestion performed with 100 µg/ml PK at 37°C for 1 h (lanes 3, 4, and 7). Positive control was from a transgenic mouse expressing wild type HuPrP-129MV challenged with the same CJD inoculum (lanes 1and 2). An uninoculated 117VV Tg31 mouse brain is shown in lanes 8 and 9. (B) Brain homogenate of a 117VV Tg31 mouse inoculated with iatrogenic CJD prions that died without clinical disease at 804 days post-infection, showing weakly detectable PrPSc partially resistant to harsh PK digestion of 100 µg/ml for 1 hour at 37°C (lane 4) compared to the same control as in Figure 3A (lanes 1 and 2). Brain homogenate of a mouse killed relatively early at 294 days post-inoculation, shows no detectable PrPSc (lane 3). (C) Immunoblots of brains of five separate 117VV Tg31 mice all inoculated with the same GSS A117V patient brain homogenate showing the presence of PrPC, PrPSc and 8 kDa PrP fragment following harsh PK digestion at 100 µg/ml PK at 37°C for 1 hour (lanes 3–7). Under these conditions PBS-inoculated age-matched control 117VV Tg31 mouse brain shows only residual PrPC signal on long exposure (lane 8). Brain homogenate of a mouse culled relatively early at 188 days post-inoculation, compared to the group mean survival post-inoculation of >616 days, showed no detectable PrPSc (lane 3). One 117VV Tg31 mouse was clinically sick at 673 days post-infection, and its brain sample shows complete digestion of PrPC and the presence of classical PrPSc (lane 7, denoted by *) confirming adequacy of the PK digestion conditions. (D) Immunoblotting was repeated for all samples shown in lanes 4–7 of Figure 3C. These samples had undergone only one further freeze–thaw cycle before PK digestion. Compared with the readily detectable abnormal PrP signals seen in Figure 3C, only one sample (denoted by *) now showed the presence of classical PrPSc but at reduced signal strength, and only after using PK at a reduced concentration of 10 µg/ml. In other samples, only an 8 kDa PrP fragment could be variably detected but after using reduced PK concentrations (see Figure S2B lanes 3 and 4). (E) Immunoblot showing only the 8 kDa PrP fragment associated with A117V-challenged 117VV Tg30 mouse brains analysed with 50 µg/ml PK at 37°C for 1 hour (lanes 7 and 8), whereas PrP in brain homogenates of PBS-challenged Tg30 mice (lanes 3–6) and uninoculated age-matched Tg30 mouse brain (lane 9) is completely digested under the same conditions. Positive control in lanes 1 and 2 is brain homogenate of a transgenic mouse expressing wild type HuPrP (129MM Tg35) that was challenged with classical CJD.