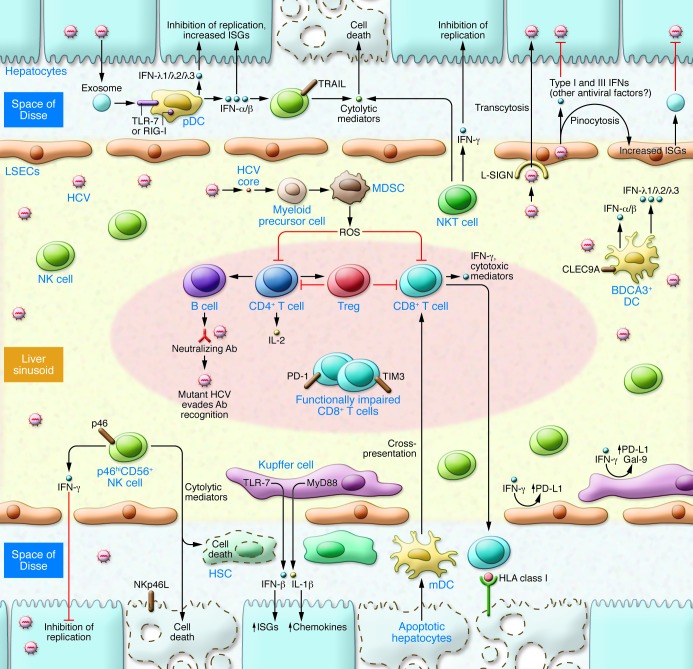
Figure 2. Immune response to HCV infection within the liver.
Viral RNA is transferred to pDCs, triggering robust production of IFNs that inhibit HCV replication in hepatocytes. pDCs produce more type I (IFN-α and IFN-β) IFNs, whereas BDCA3+ DCs produce more type III IFNs with HCV infection and do not require direct cell-to-cell contact. NK and NKT cells comprise a large proportion of intrahepatic lymphocytes, mediating antiviral functions through a combination of IFN type II (IFN-γ) production and cytolytic function. IFNα-induced TRAIL is associated with the control of HCV (78, 82). Hepatic accumulation of NKp46hi NK cells is associated with lower viral replication and attenuated fibrosis (78). KCs phagocytose HCV, leading to the induction of innate immune (IFN-β) as well as inflammatory (IL-1β) responses. HCV core protein inhibits type I IFN responses (89) and also drives proinflammatory responses, augmenting processes that result in liver fibrosis (87). IFN-γ induces KC upregulation of Gal-9 and PD-L1, inhibitory ligands that promote T cell dysfunction. LSECs can pinocytose viral particles and produce a broad array of IFNs. Multispecific and polyfunctional CD4+ T (Th) cells provide “help” for clonal expansion of B cells and CTLs required for spontaneous viral control. Early expression of CD127, IL-2 production, development of neutralizing Abs, and HCV-specific CTL cells contribute to immune response (148, 149). PD-1 and TIM3 demarcate functionally impaired CTLs. Moreover, CD33+ myeloid–derived suppressor cells (150) and FOXP3+ Tregs (10, 151) attenuate T cell responses and immune-mediated liver injury.