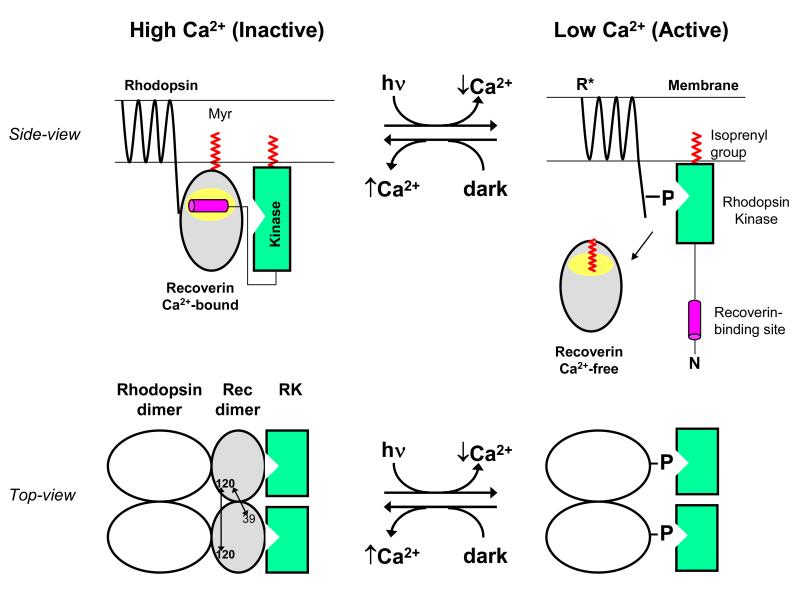
Figure 6.
Schematic model showing functional role of Ca2+-induced dimerization of recoverin. A side-view of recoverin on the disk membrane is shown in the upper panel and a top-view of dimeric recoverin is shown in the lower panel. Myristoylation (red) targets Ca2+-bound recoverin to the membrane surface (upper panel). The Ca2+-bound recoverin dimer binds to the N-terminal helix of rhodopsin kinase (magenta), forming a 2:2 complex on the membrane surface that blocks phosphorylation of dimeric rhodopsin (lower panel). Intermolecular DEER distances for the recoverin dimer are indicated by double-headed arrows. Light activation leads to a lowering of cytosolic Ca2+, causing conformational changes in recoverin that promote dimer dissociation, sequester the covalently attached myristoyl group, and disrupt binding to rhodopsin kinase (RK). Ca2+-free monomeric recoverin then dissociates from the membrane surface, allowing RK to phosphorylate the C-terminal tail of light-excited rhodopsin (R*).