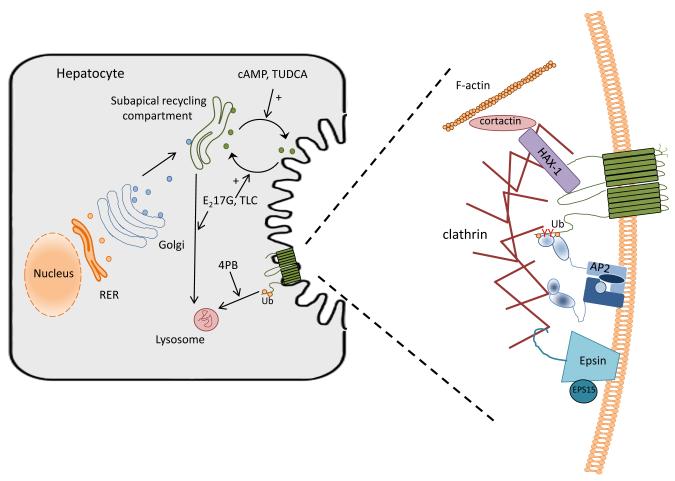
Fig 1.
Biosynthesis, trafficking and regulation of BSEP in the hepatocyte. (A) After post-translational modification in the Golgi, BSEP is trafficked to a subapical recycling compartment. Traffic to and from this endosomal compartment is highly regulated through various kinase pathways (Roma et al., 2008). Notably, cAMP and tauroursodeoxycholate treatment result in increased movement to the canalicular plasma membrane, whereas estradiol 17-glucuride and taurolithocholate treatment lead to increased endocytosis back to the recycling endosome and, perhaps eventually, to the lysosome for degradation. Phenylbutyrate (PB) may act by altering the rate of ubiquitination (Ub) and lysosomal degradation of BSEP. (B) Internalization of BSEP from the plasma membrane is a clathrin-dependent process involving many interacting partners. AP-2 has been shown to bind to a tyrosine containing motif in the C-terminus of Bsep which allows for endocytosis by a clathrin dependent mechanism (Hayashi et al., 2012; Lam et al., 2012). 4-PB has been shown to decrease the expression of the α-adaptin subunit of AP2, thus altering the ability of AP2 to interact with BSEP and decreasing endocytosis of the transporter (Hayashi et al., 2012). Ubiquination of the C-terminus of BSEP has also been shown to influence the cell surface expression of the transporter (Hayashi and Sugiyama, 2009). HAX-1 binds to the NBD domain of BSEP and with cortactin and has been shown to be involved in BSEP internalization from the plasma membrane (Ortiz et al., 2004). Epsin and EPS15 are important to the formation of the clathrin coated vesicle and, thus can influence the endocytosis of BSEP from the canalicular membrane (Ortiz et al., 2004). Regulation of these complexes through signaling pathways and by such compounds as phenylbutyrate may provide mechanisms for therapeutic intervention in various forms of cholestasis.