Abstract
We found that carprofen and meloxicam under 3 environmental conditions (ambient dark, ambient light, and 4 °C) remained stable for at least 7 d. We then evaluated the oral pharmacokinetics of meloxicam (20 mg/kg) and carprofen (10 mg/kg) in male C57BL/6 mice after oral gavage or administration in the drinking water. Mice did not drink meloxicam-medicated water but readily consumed carprofen-medicated water, consuming an average of 14.19 mL carprofen-medicated water per 100 g body weight daily; mice drank more during the dark phase than during the light phase. Plasma analyzed by HPLC (meloxicam) and tandem mass spectrometry (carprofen) revealed that the peak meloxicam and carprofen concentrations were 16.7 and 20.3 μg/mL and occurred at 4 and 2 h after oral gavage, respectively. Similar blood levels were achieved after 12 h access to the carprofen-medicated water bottle. At 24 h after oral gavage, the drugs were not detectable in plasma. Meloxicam plasma AUC, elimination half-life, apparent volume of distribution, and apparent oral clearance were 160.4 mg/L × h, 7.4 h, 0.36 L/kg, and 0.125 mL/h × kg, respectively. Carprofen plasma AUC, elimination half-life, apparent volume of distribution, and apparent oral clearance were 160.8 mg/L × h, 7.4 h, 0.42 L/kg, and 0.062 mL/h × kg, respectively. No gross or microscopic evidence of toxicity was seen in any mouse. Our findings indicate that carprofen can be administered in drinking water to mice and that medicated water bottles should be placed 12 to 24 h prior to painful procedures.
Abbreviation: COX, cyclooxygenase
The provision of appropriate and effective analgesia for research rodents is a constant challenge for veterinary care personnel.1,22,53,54 Several barriers exist that may hinder the administration of sufficient analgesia, including appropriate training for administering analgesics, the possibility for inducing handling stress, interstrain and interindividual variability in response to and metabolism of a given analgesic substance, and the different levels of analgesic required after any one of the numerous procedures or experimental conditions that any given research mouse may undergo.14 Furthermore, little information is available in the literature regarding the pharmacokinetics of commonly used NSAID in laboratory mice or after alternative routes of administration. Information regarding method of administration, dose ranges, and dosing intervals is often extrapolated from other species; however, the extent of interspecies variability is often unknown.
Carprofen is a NSAID that is derived from propionic acid and has antiinflammatory, antipyretic, and analgesic properties. The compound has a chiral center at the α-carbon position and exists as a racemic mixture composed of R (–) and S (+) enantiomers,47 which is a complicating factor in pharmacokinetic investigations. In all species studied to date, the R enantiomer is less potent than is the S enantiomer, and there is minimal conversion between enantiomers in vivo.26 As with other NSAID, the positive pharmacologic effects of carprofen arise through species-specific differential inhibition of cyclooxygenase (COX) isomers. The pharmacokinetics of carprofen have been evaluated in several mammalian species, including rats,21,24 cows,10,27 dogs,33 cats,48 sheep,7,56 horses,11,27,34,46 rabbits,18 and humans.47 Most9,20,23,33,46,54 of these studies evaluated pharmacokinetic parameters after intravenous or subcutaneous administration; other studies33,47 evaluated the pharmacokinetics of carprofen after oral administration. Evidence regarding the relative COX2 selectivity of carprofen is inconsistent throughout the literature,23 and in vitro studies have suggested that carprofen is a nonselective inhibitor of COX2 in horses but a selective COX2 inhibitor in cats.3 These findings serve to further emphasize the potential for interspecies variability in the pharmacokinetics of carprofen and the need for species-specific dosage information.
Meloxicam is an enolic acid NSAID derivative that has antiinflammatory, analgesic, and antipyretic properties.13 Its antiinflammatory action is achieved through preferential inhibition of COX2, which may contribute to reducing the gastrointestinal side effects that may be seen with other NSAID. Meloxicam has a long half-life in many species, thereby supporting once-daily administration and making the drug an attractive veterinary analgesic.13 The pharmacokinetics of meloxicam have been evaluated in several mammalian species, including rats,43 horses,45,52 cows,37 goats,20,44 sheep,44 pigs,15,16 dogs,4,36,58 donkeys,28 chickens,2 humans,42 and some exotic and wild species (for example, camels,55 iguanas,12 vultures38).
Both carprofen and meloxicam typically are administered to mice subcutaneously, but there are anecdotal reports of the provision of NSAID in the drinking water. This method of administration is an attractive option, because it potentially could ensure that adequate and stable blood drug levels are achieved throughout a period of analgesic need and would remove the handling stress associated with subcutaneous injection or oral gavage.8,19,35 To date, no data have been published to support this route of administration for NSAID to mice. Before adopting this practice, it is important to determine whether meloxicam and carprofen are stable when diluted in water, whether rodents consume medicated water, and whether blood drug levels obtained after this route of administration are comparable to those of other methods of administration. The current study evaluated the stability of carprofen and meloxicam in water under various environmental conditions and assessed the palatability and oral pharmacokinetics of both compounds when administered in the drinking water and by oral gavage. In addition, mice were evaluated for evidence of acute NSAID-related toxicity of gastrointestinal, hepatic, and renal tissues.
Materials and Methods
Animals.
Male 6-wk-old C57BL/6J male mice (Mus musculus) were purchased from Charles River Laboratories (St Constant, Quebec, Canada). Mice weighed an average of 20.5 g (range, 17.2 to 23.4 g) and were housed in groups of 4 in standard polycarbonate cages on corncob bedding (Teklad Corn Cob Bedding, Harlan Teklad, Madison, WI). Each cage contained a clear polycarbonate hut, a cotton nesting square, and small handful of crimped paper. Mice were acclimated to a reversed 12:12-h light:dark cycle for 7 d prior to study initiation, and food (Teklad Global 14% Protein Rodent Maintenance Diet, Harlan Teklad) and tap water were provided ad libitum. Vendor health surveillance reports indicated that animals were free from mouse adenovirus, mouse hepatitis virus, mouse parvoviruses, mouse rotavirus, mouse norovirus, Theiler murine encephalomyelitis virus, Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Mycoplasma pulmonis, Salmonella spp., Helicobacter spp., Klebsiella spp., Pasteurella spp., Staphylococcus aureus, and Streptococcus spp., ectoparasites, endoparasites, and enteric protozoa. The University of Guelph Animal Care Committee approved the animal use protocol, and the facility and procedures are in compliance with the Animals for Research Act of Ontario and the guidelines of the Canadian Council on Animal Care.6,39
Experimental design.
We evaluated the stability of diluted injectable solutions of carprofen and meloxicam over a 7-d period. We used injectable solutions of both NSAID for this study because the low concentration of commercially available meloxicam suspensions was cost-prohibitive for use in the drinking water of large numbers of mice.
For the pharmacokinetic study, 176 mice (n = 4 per cage) were randomized into 1 of 4 treatment groups: 20 mg/kg meloxicam by oral gavage, 20 mg/kg meloxicam by water bottle, 10 mg/kg carprofen by oral gavage, or 10 mg/kg carprofen by water bottle. At 0, 5, 15, 30, and 60 min and 2, 4, 8, 12, 24, and 36 h after administration, 4 mice were anesthetized by using isoflurane (Aerrane, Baxter, Mississauga, Ontario, Canada) in oxygen, and exsanguinated by cardiocentesis. Blood was collected into EDTA- (carprofen) or heparin- (meloxicam) coated tubes (Sarstedt, St Leonard, Quebec, Canada).
Preparation of NSAID stability samples.
To evaluate the stability of meloxicam when diluted, 2.34 mL meloxicam (5 mg/mL; Metacam injectable, Boehringer Ingelheim, Burlington, Ontario, Canada) was added to 87.66 mL reverse-osmosis–purified water to yield a final solution concentration of 0.130 mg/mL. This solution was divided into 3 glass flasks, and one each was stored at ambient light, ambient dark, and 4 °C dark conditions. A 1-mL sample was collected from each flask daily for 7 d and frozen at −80 °C until further analysis.
For carprofen solutions, 0.12 mL carprofen (50 mg/mL; Rimadyl injectable, Pfizer Canada, St Laurent, Quebec, Canada) was added to 89.88 mL of reverse-osmosis–purified water to make a final solution concentration of 0.067 mg/mL. Solutions were stored and collected as described for meloxicam. Because of cost constraints, only samples from days 1, 3, and 7 after preparation were analyzed for carprofen concentration.
Preparation of NSAID dosing solutions.
An average mouse body weight for each treatment group was obtained the day prior to study initiation for each drug and administration method, and doses were based on average body weight. For groups dosed by water bottle, dose concentrations were based on the assumption that mice would consume 15 mL per 100 g body weight every 24 h.17 Bottles containing the NSAID in water were placed on cages at time 0; bottles containing untreated water were removed. The final solutions contained 0.13 mg/mL meloxicam or 0.067 mg/mL carprofen, with reverse-osmosis–purified water as the diluent. For oral gavage studies, mice were gavaged by using a volume of 5 mL/kg and a 22-gauge stainless steel gavage needle. All solutions were prepared immediately prior to administration. The final meloxicam solution was noted to have a mild, acrid odor, whereas the carprofen solution had no odor. Water consumption was calculated on a per-animal basis by subtracting the weight of the water bottle at the times of blood collection from the weight of the water bottle before drug administration. This value was then divided by the number of mice per cage.
Determination of plasma meloxicam levels.
After blood collection, samples were placed on ice immediately and then centrifuged to separate plasma, which was frozen at −80 °C until further analysis. Meloxicam plasma concentrations were determined by HPLC. Briefly, samples were prepared by combining 100 μL plasma, 10 μL of the internal standard solution (piroxicam, 10 μg/mL in methanol; Sigma-Aldrich, Oakville, Quebec. Canada), 10 μL 1 N HCl, and 1 mL diethyl ether. The mixture was vortexed and centrifuged, and the upper layer was collected and evaporated under a constant flow of nitrogen. The residue was reconstituted in 100 μL of the mobile phase, and 50 μL was injected into the analytical column.
The Liquid Phase Separation and Assay Facility (Ontario Veterinary College, University of Guelph, Canada) analyzed the samples. A chromatograph (Waters Alliance 2695, Mississauga, Ontario, Canada) with a photodiode array detector (model 2996, Waters) was connected to Empower 2 software (Waters) for data collection and processing. An analytical column (50 mm × 4.6 mm; inner diameter, 2.5 μm; Sunfire C18, Waters, Wexford, Ireland) was connected to a guard column (4 mm × 3.0 mm; Security Guard C18, Phenomenex, Torrance, CA), and isocratic chromatographic separation was conducted by using a mobile phase containing acetonitrile–water–acetic acid (60:40:1, v/v/v) at a flow rate of 1 mL/min.
Calibration curves were prepared by spiking 100 μL untreated (control) mouse plasma with 10 μL meloxicam (Sigma-Aldrich, Oakville, Ontario, Canada) to create working solutions (5, 10, 25, 50, and 75 μg/mL) and 10 μL of piroxicam internal standard solution (10 μg/mL). The limit of detection was 0.25 μg/mL, on the basis of 3 times the signal-to-noise ratio at the time of analyte elution. The limit of quantitation for the assay was 0.5 μg/mL.
Determination of plasma carprofen levels.
Because of the expense and difficulty of acquiring enantiomer-specific data, we measured total plasma carprofen levels in this study. After blood collection, samples were placed on ice, blood was centrifuged, and plasma was collected and frozen at −80 °C until further analysis. Prior to analysis, samples were prepared by extracting 0.1 mL plasma with 1 mL 1% ascorbic acid in 0.1 N HCl and 10 mL ethyl acetate. An aliquot (8 mL) of this extract was evaporated to dryness by using a nitrogen evaporator (N-Evap Analytical Evaporator, Organomation Association, Berlin, MA) set at 60 °C. The residue was redissolved in 0.2 mL of the internal standard solution, 1 μg/mL d3-carprofen in 50% methanol:water (v/v; Toronto Research Chemicals, Ontario, Canada).The mixture was vortexed and then filtered through a 0.22-μm HPLC filter (Millipore, Milford, MA) into a glass autosampler vial for analysis.
The extract was analyzed by using an HPLC system (Microm BioResources, Auburn, CA) coupled with a hybrid triple quadrupole–linear ion trap mass spectrometer (model 4000 Q TRAP, AB SciEx, Toronto, Ontario, Canada). The HPLC column was a 20 × 2 mm × 3 mm C18 column2 (Luna C18, Phenomenex), with a mobile phase containing 0.01 M ammonium acetate in 0.1% formic acid in water (A solution) and 0.01M ammonium acetate in 0.1% formic acid in methanol (B solution) at a flow rate of 200 μL/min under a linear gradient of 50% B to 95% B over 7 min. The analysis for carprofen was completed by the California Animal Health and Food Safety Laboratory System Toxicology Laboratory (UC Davis School of Veterinary Medicine, Davis, CA).
HPLC methods were validated prior to assay, and calibration curves were prepared by spiking 1 mL untreated (control) mouse serum with 50 μL of 10 μg/mL carprofen standard (Toronto Research Chemicals, ON) and analyzing by using the method described. The limits of quantitation for the assay were 0.50 μg/mL and 1.3 μg/mL for drinking water and gavage samples, respectively.
Microscopic evaluation of tissues.
After euthanasia by isoflurane–oxygen anesthesia and exsanguination, liver, lung, kidneys, stomach, and duodenum were removed from each animal and fixed in 10% buffered formalin. Tissues were trimmed, embedded in paraffin, sectioned, and stained routinely with hematoxylin and eosin for microscopic evaluation. Slides were evaluated without a priori knowledge of animal treatment.
Data analyses.
Data are reported as mean ± SEM. One-way ANOVA was used to determine significant differences in drug concentration in water between the 3 tested environmental conditions and by sampling day. A significant effect of day was evaluated further by using individual Student t tests. For all statistical analyses, only significant results are reported, and the α level was set as a P value of less than 0.05. Statistical analyses were performed by using SPSS 13.0 software (SPSS, Chicago, IL).
Determinations of maximal plasma meloxicam and carprofen concentrations and time to maximal concentration were determined by direct observation of data. Pharmacokinetic parameters (plasma AUC; elimination rate constant; elimination half-life; apparent volume of distribution; and apparent oral clearance) were determined by using noncompartmental analyses. The AUC was calculated by using the trapezoidal rule and was extrapolated to infinity. The elimination rate constant was calculated by determining the rate of change (elimination) of the logarithmic linear regression of the plasma concentration–time curve. The elimination half-life was calculated by dividing 0.693 by the elimination constant. Apparent volume of distribution was calculated by dividing the dose of drug administered by the product of the elimination constant and the AUC. Note that the apparent volume of distribution is estimated as the volume of distribution divided by the bioavailability factor (from 0 to 1), because the exact amount of drug that enters the systemic circulation after oral administration is unknown.51 Apparent oral clearance was calculated by dividing the dose by the AUC.
Results
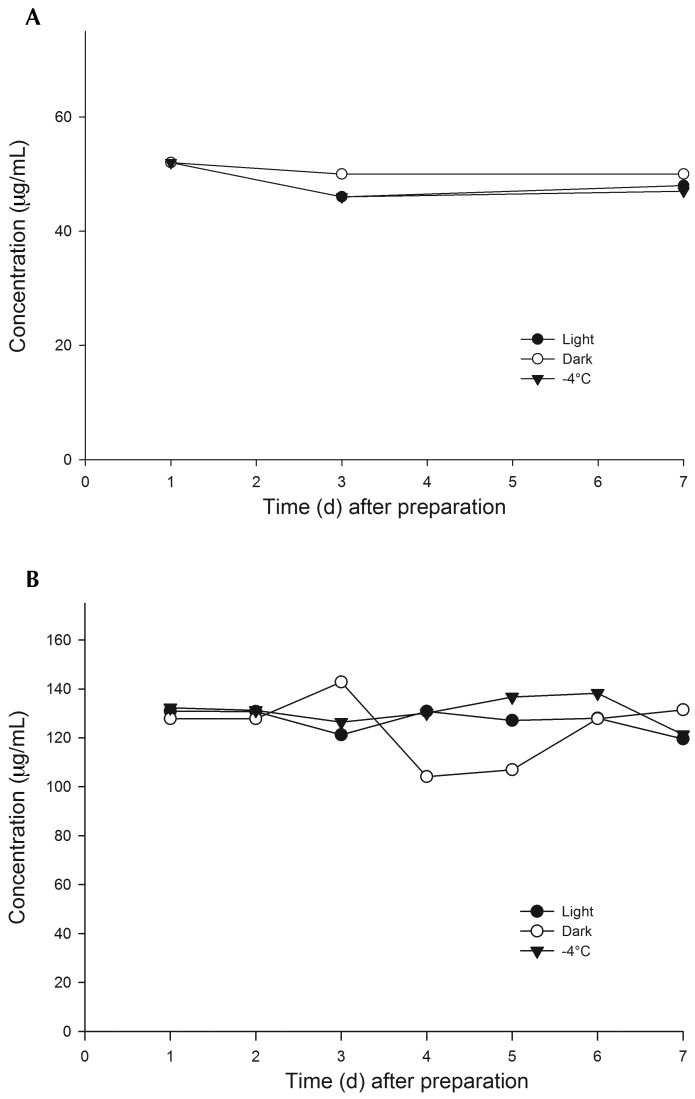
Both injectable meloxicam (Figure 1 A) and carprofen (Figure 1 B) were stable for 7 d when diluted in reversed-osmosis–purified water and held under ambient light, ambient dark, and 4 °C conditions. Analyte concentrations of solutions on a given day did not differ according to the environmental conditions under which the solutions were held or between days 1 and 7. Meloxicam concentration did not differ by day. There was a significant effect of day on carprofen concentration, which occurred between days 0 and 3 (P = 0.008) and days 0 and 7 (P = 0.004).
Figure 1.
Stability of injectable (A) meloxicam (0.013 mg/mL) and (B) carprofen (0.067 mg/mL) solutions diluted in reverse-osmosis–purified water and held in ambient light, ambient dark, and refrigerated dark (4 °C) environmental conditions for 7 d. Both NSAID were stable under all conditions tested for 7 d.
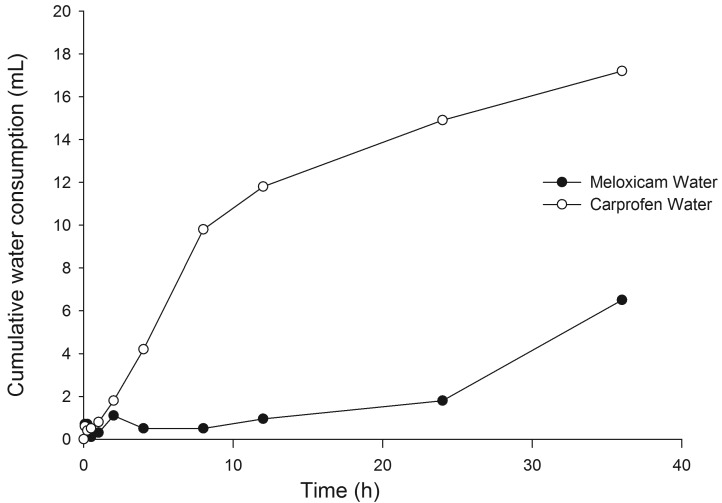
In general, mice consumed little or no meloxicam-treated drinking water over the 36-h test period (Figure 2). In contrast, mice readily consumed carprofen-treated drinking water over the 36-h study, drinking an average of 14.2 mL per 100 g body weight every 24 h (Figure 2). As expected, mice consumed more carprofen-treated drinking water during the dark phase (17.5 mL per 100 g body weight every 24 h during 0 to 12 h and 24 to 36 h) than during the light phase (7.7 mL per 100 g body weight every 24 h during 12 to 24 h). Furthermore, mice drank more carprofen-treated drinking water during the first dark phase (29.2 mL per 100 g body weight every 24 h) than during the second dark phase (5.7 mL per 100 g body weight every 24 h).
Figure 2.
Consumption of NSAID in water over 36 h. Mice readily consumed carprofen in water but refused to drink meloxicam in water. Mice drank an average of 14.1 mL carprofen solution per 100 g body weight in 24 h. Mice consumed more water during the dark phase (0 to 12 h and 24 to 36 h in this study) than during the light phase.
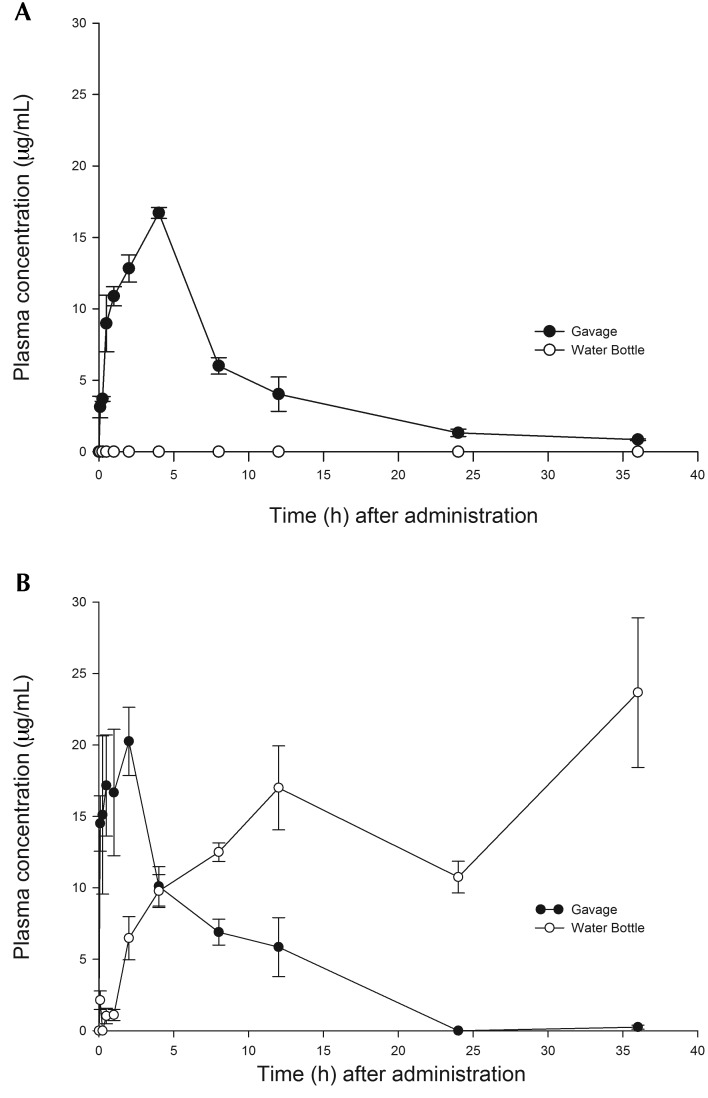
After a single oral dose of 20 mg/kg meloxicam by gavage, the peak plasma concentration of 16.7 ± 0.4 μg/mL was achieved at 4 h after administration (Figure 3 A). After a single oral dose of 10 mg/kg carprofen by gavage, the peak concentration of 20.3 ± 2.4 μg/mL was reached at 2 h after administration, whereas the peak plasma concentration of 17.0 ± 2.9 μg/mL was achieved after 12 h of exposure to the carprofen-medicated water bottle (Figure 3 B). For both NSAID, plasma concentrations after oral gavage rapidly declined to near undetectable levels (meloxicam, 1.3 ± 0.3 μg/mL; carprofen, 0 μg/mL) at 24 h after administration. The plasma carprofen concentration continued to increase with continued exposure to the medicated water bottle. Table 1 provides the pharmacokinetic parameters for meloxicam and carprofen after oral gavage.
Figure 3.
Plasma drug concentrations after a single oral dose (gavage) or after constant dosing through the drinking of water containing (A) meloxicam (20 mg/kg) and (B) carprofen (10 mg/kg). After 12 h of exposure to the aqueous carprofen solution, mice achieved comparable plasma carprofen levels to those obtained after oral gavage. Levels were sustained after administration in the drinking water.
Table 1.
Pharmacokinetic parameters after oral gavage of meloxicam (20 mg/kg) or carprofen (10 mg/kg) to male C57BL/6 mice (n = 6 per group)
| Meloxicam | Carprofen | |
| Peak plasma concentration (µg/mL) | 16.7 ± 0.4 | 20.3 ± 2.4 |
| Time to peak plasma concentration (h) | 4.0 ± 0.0 | 2.0 ± 0.4 |
| Area under the concentration–time curve (mg/L×h) | 160.4 ± 2.8 | 160.8 ± 14.6 |
| Elimination half-life (h−1) | 7.4 ± 2.0 | 7.4 ± 4.6 |
| Apparent volume of distribution (L/kg) | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Apparent oral clearance (mL/kg/h) | 0.12 ± 0.00 | 0.06 ± 0.00 |
The half-lives of both compounds were similar, the apparent volume of distribution of carprofen was slightly greater compared with that obtained for meloxicam (Table 1), and the clearance of carprofen was half that of meloxicam. Bioavailability issues are unlikely to have influenced clearance and volume of distribution, because both compounds are almost 100% bioavailable in mice (meloxicam) and in other species (carprofen) after oral dosing.5,50,51
There were no gross or microscopic changes indicative of NSAID-related toxicity in any tissue examined.
Discussion
We have demonstrated that mice will readily consume carprofen-treated drinking water and that the maximal plasma concentration of 10-mg/kg doses at 2 h after gavage and at 12 h after the addition of treated water bottles to cages are similar, although the plasma levels of carprofen are much more sustained when administered in the drinking water than by oral gavage. Dilutions of carprofen in water are stable for at least 7 d in the light or dark at room temperature, suggesting that this administration technique could readily be used in a vivarium as a replacement for individual dosing. Furthermore, despite similar stability findings for both drugs, mice did not consume injectable meloxicam when it was diluted in water. We did not evaluate whether mice would consume the oral suspension of meloxicam when diluted in water because this route would be cost-prohibitive for providing analgesia to large numbers of mice on a study and because the commercially available oral suspensions are too dilute to reach effective concentrations when administered in the drinking water. Meloxicam is clearly an effective analgesic compound when parenterally administered to mice postoperatively.14,25,41,57 However, meloxicam plasma levels are not sustained, and repeated injections are required to provide adequate analgesia for many surgical procedures, as demonstrated in a recent study in which mice exhibited signs of pain for 24 to 36 h after laparotomy.30 Repeated handling is stressful to mice;8,19,35 therefore, methods that minimize handling yet ensure adequate analgesic coverage represent a refinement in the care of these animals.
The current study evaluated total plasma levels of carprofen, and as such, comments about the pharmacokinetic parameters obtained are somewhat limited. The comparative potency and metabolism of the R- and S- enantiomers of carprofen have not been reported for mice, and enantiomer concentrations were not evaluated in the current study. Within a given species, the ratio of the enantiomers is constant, but whether enantiomer shifting occurs when carprofen is diluted in aqueous solutions is unknown.29 Meloxicam and carprofen appear to have similar pharmacokinetics in mice, and on the basis of the total clearance rate, carprofen has a low to moderate blood clearance rate, indicating that it is a suitable candidate for oral administration in this species.49 The rapid time to peak plasma concentration and modest half-life of carprofen in mice suggest that a dosing interval more frequent than once daily may be needed to maintain plasma drug levels. This conclusion has been indirectly supported by a recent study, which showed that the analgesic effect of carprofen at 20 mg/kg SC in mice after laparotomy was unlikely to last beyond 8 h.30 Tissue drug levels and efficacious plasma drug levels are unknown for carprofen in mice and are required for the determination of an appropriate interval for oral dosing. The plasma drug levels seen after the provision of carprofen-treated drinking water may indicate accumulation of carprofen over time, potentially related to overwhelming hepatic metabolism through repeated dosing or through enterohepatic recycling and accumulation, which can be considerable for carprofen in other species.9,40 The total plasma levels of carprofen continued to increase when the water bottle was left on the cage to 36 h. Although this increase may prompt concerns regarding potential toxicity, no gross or microscopic changes suggestive of acute toxicity were noted in any animal at any time point to suggest acute toxicity, and this increase may just reflect the maintenance of a therapeutic plasma carprofen concentration over a longer period with this method of dosing. Given the stereoselective effects of the enantiomers, the total carprofen plasma levels we obtained likely overestimate the plasma levels of the more efficacious S enantiomer.
The LD50 for oral carprofen is 30 mg/kg daily in nulliparous rats and 19 mg/kg daily in lactating rats.31 Similar to dogs, rats demonstrate significantly different carprofen pharmacokinetics than those in mice, with a terminal half-life as long as 40 h and with increased sensitivity to toxic effects in lactating animals.32 The LD50 for oral carprofen has not been reported for mice. Additional studies are required to determine the extent of accumulation that would occur over time should mice receive access to carprofen-medicated water bottles for longer than 36 h and to characterize any associated pathologic changes. In addition, correlative efficacy studies are required to determine therapeutic plasma carprofen levels in mice.
Current knowledge about murine behavior predicts that mice will drink more water during the dark phase than the light phase,17 as was seen in the current study. Interestingly, significantly less carprofen-medicated water was consumed during the second dark phase than the first. The amount consumed during the second dark phase was also less than that consumed during the light phase. The reason for this difference is unknown. Carprofen-treated drinking water may be very palatable to mice, and mice may have overhydrated during the first 12 h of access, resulting in lower consumption subsequently. Unaccounted leakage of bottles when they are inverted onto cage lids is typically minimal (a few drops) and is unlikely to account for the difference we noted between the 2 dark periods. The overall consumption was almost precisely that predicted over a 24-h period (15 mL/100 g body weight), suggesting that the difference in the volume of water consumed was not due to a sudden change in palatability. Anecdotally, at our facility, carprofen-treated drinking water has been given to female mice with litters in an attempt to provide analgesia to pups prior to painful procedures, such as ear notching. Whether female mice or mice of different strains or ages consume the medicated water at a similar rate to that of the male mice in the current study or whether carprofen levels in the milk are similar to plasma levels are unknown. Additional studies are required to investigate whether carprofen-treated water is more palatable to mice than is nonmedicated water and whether mice of different strains or sex consume carprofen-treated water at similar rates.
The oral pharmacokinetics of meloxicam (10 mg/kg) in male and female mice have previously been investigated.5 Peak plasma concentrations of 18.1 and 20.7 mg-eq/L were achieved at 0.7 and 0.6 h after administration for male and female mice, respectively. Our study achieved a similar peak plasma concentration; however, the time to peak plasma concentration was approximately 3 times that seen previously.5 Unlike the mice in the previous study, our mice were not fasted prior to dosing, and this variation may account for much of the difference in time to peak plasma concentration. As expected, the AUC in the current study was much larger, given that our dose was twice that used in the earlier study.5 Importantly, the previous study demonstrated good (94%) oral bioavailability of the drug, indicating that the apparent clearance and volume of distribution values obtained in the current study likely are very close to the actual values.
We evaluated the pharmacokinetics of meloxicam and carprofen after oral dosing. However, the clinical efficacy of the drugs given at these doses and by this route of administration has not yet been assessed. This information gap warrants further study.
In summary, both meloxicam and carprofen are stable in aqueous solutions when held for 7 d in dark, light, and cold environmental conditions. Single doses of meloxicam or carprofen in mice are cleared relatively rapidly and likely require greater than once-daily dosing to maintain therapeutic blood drug levels. Drinking water containing carprofen (10 mg/kg) is palatable to mice, whereas aqueous solutions of injectable meloxicam (20 mg/kg) are highly unpalatable. To achieve peak blood drug levels similar to those obtained after oral gavage, carprofen-containing water bottles should be placed on mouse cages at least 12 h prior to painful procedures.
Acknowledgments
This project was funded by an AALAS Grant for Laboratory Animal Science (GLAS). We thank Ms Charlotte Sir for technical assistance.
References
- 1.Anil SS, Anil L, Deen J. 2002. Challenges of pain assessment in domestic animals. J Am Vet Med Assoc 220:313–319 [DOI] [PubMed] [Google Scholar]
- 2.Baert K, De Backer P. 2002. Disposition of sodium salicylate, flunixin, and meloxicam after intravenous administration in broiler chickens. J Vet Pharmacol Ther 25:449–453 [DOI] [PubMed] [Google Scholar]
- 3.Brideau C, Van Staden C, Chan CC. 2001. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs, and cats. Am J Vet Res 62:1755–1760 [DOI] [PubMed] [Google Scholar]
- 4.Budsberg SC, Cross AR, Quandt JE, Pablo LS, Runk AR. 2002. Evaluation of intravenous administration of meloxicam for perioperative pain management following stifle joint surgery in dogs. Am J Vet Res 63:1557–1563 [DOI] [PubMed] [Google Scholar]
- 5.Busch U, Schmid J, Heinzel G, Schmaus H, Baier J, Huber C, Roth W. 1998. Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos 26:576–584 [PubMed] [Google Scholar]
- 6.Canadian Council on Animal Care. [Internet]. 1993. CCAC guide to care and use of experimental animals, vol 1. [Cited 18 August 2013] Available at: http://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf.
- 7.Cheng Z, Nolan A, Monteiro A, McKellar Q. 2003. Enantioselective pharmacokinetics and cyclooxygenase inhibition of carprofen and carprofen enantiomers in sheep. J Vet Pharmacol Ther 26:391–394 [DOI] [PubMed] [Google Scholar]
- 8.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 9.Clark TP, Chieffo C, Huhn JC, Nimz EL, Wang C, Boy MG. 2003. The steady-state pharmacokinetics and bioequivalence of carprofen administered orally and subcutaneously in dogs. J Vet Pharmacol Ther 26:187–192 [DOI] [PubMed] [Google Scholar]
- 10.Delatour P, Foot R, Foster AP, Baggot D, Lees P. 1996. Pharmacodynamics and chiral pharmacokinetics of carprofen in calves. Br Vet J 152:183–198 [DOI] [PubMed] [Google Scholar]
- 11.Delatour P, Garnier F, Maire R. 1996. Enantioselectivity in the excretion of glucuronides of carprofen in man, dogs, and horses. Bull Acad Natl Med 180:1565–1572 [PubMed] [Google Scholar]
- 12.Divers SJ, Papich M, McBride M, Stedman NL, Perpinan D, Koch TF, Hernandez SM, Barron GH, Pethel M, Budsberg SC. 2010. Pharmacokinetics of meloxicam following intravenous and oral administration in green iguanas (Iguana iguana). Am J Vet Res 71:1277–1283 [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt G, Homma D, Schlegel K, Utzlnann R, Schnitzler C. 1995. Antiinflammatory, analgesic, antipyretic, and related properties of meloxicam, a new nonsteroidal antiinflammatory agent with favourable gastrointestinal tolerance. Inflamm Res 44:423–433 [DOI] [PubMed] [Google Scholar]
- 14.Flecknell PA. 2009. Laboratory animal anaesthesia: a practical introduction for research workers and technicians, 3rd ed. San Diego (CA): Academic Press. [Google Scholar]
- 15.Fosse TK, Haga HA, Hormazabal V, Haugejorden G, Horsberg TE, Ranheim B. 2008. Pharmacokinetics and pharmacodynamics of meloxicam in piglets. J Vet Pharmacol Ther 31:246–252 [DOI] [PubMed] [Google Scholar]
- 16.Fosse TK, Spadavecchia C, Horsberg TE, Haga HA, Ranheim B. 2011. Pharmacokinetics and pharmacodynamic effects of meloxicam in piglets subjected to a kaolin inflammation model. J Vet Pharmacol Ther 34:367–375 [DOI] [PubMed] [Google Scholar]
- 17.Harkness J, Turner PV, Vandewoude S, Wheler CL. 2010. Harkness and Wagner's biology and medicine of rabbits and rodents, 5th ed. Ames (IA): Wiley-Blackwell. [Google Scholar]
- 18.Hawkins MG, Taylor IT, Craigmill AL, Tell LA. 2008. Enantioselective pharmacokinetics of racemic carprofen in New Zealand white rabbits. J Vet Pharmacol Ther 31:423–430 [DOI] [PubMed] [Google Scholar]
- 19.Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826 [DOI] [PubMed] [Google Scholar]
- 20.Ingvast-Larsson C, Hogberg M, Mengistu U, Olsen L, Bondesson U, Olsson K. 2011. Pharmacokinetics of meloxicam in adult goats and its analgesic effect in disbudded kids. J Vet Pharmacol Ther 34:64–69 [DOI] [PubMed] [Google Scholar]
- 21.Iwakawa S, Spahn H, Benet LZ, Lin ET. 1991. Stereoselective disposition of carprofen, flunoxaprofen, and naproxen in rats. Drug Metab Dispos 19:853–857 [PubMed] [Google Scholar]
- 22.Karas AZ. 2006. Barriers to assessment and treatment of pain in laboratory animals. Lab Anim (NY) 35:38–45 [DOI] [PubMed] [Google Scholar]
- 23.Kay-Mugford P, Benn SJ, LaMarre J, Conlon P. 2000. In vitro effects of nonsteroidal antiinflammatory drugs on cyclooxygenase activity in dogs. Am J Vet Res 61:802–810 [DOI] [PubMed] [Google Scholar]
- 24.Kemmerer JM, Rubio FA, McClain RM, Koechlin BA. 1979. Stereospecific assay and stereospecific disposition of racemic carprofen in rats. J Pharm Sci 68:1274–1280 [DOI] [PubMed] [Google Scholar]
- 25.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the mouse grimace scale. PLoS ONE 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lees P, Landoni MF. 2002. Pharmacodynamics and enantioselective pharmacokinetics of racemic carprofen in the horse. J Vet Pharmacol Ther 25:433–448 [DOI] [PubMed] [Google Scholar]
- 27.Lohuis JA, van Werven T, Brand A, van Miert AS, Rohde E, Ludwig B, Heizmann P, Rehm WF. 1991. Pharmacodynamics and pharmacokinetics of carprofen, a nonsteroidal antiinflammatory drug, in healthy cows and cows with Escherichia coli endotoxin-induced mastitis. J Vet Pharmacol Ther 14:219–229 [DOI] [PubMed] [Google Scholar]
- 28.Mahmood KT, Ashraf M. 2011. Pharmacokinetics of meloxicam in healthy donkeys. Pakistan J Zool 43:897–901 [Google Scholar]
- 29.Maire-Gauthier R, Buronfosse T, Magdalou J, Herber R, Besse S, Delatour P, Benoit E. 1998. Species-dependent enantioselective glucuronidation of carprofen. Xenobiotica 28:595–604 [DOI] [PubMed] [Google Scholar]
- 30.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49 [PMC free article] [PubMed] [Google Scholar]
- 31.McClain RM, Hoar RM. 1980. Reproduction studies with carprofen, a nonsteroidal antiinflammatory agent in rats. Toxicol Appl Pharmacol 56:376–382 [DOI] [PubMed] [Google Scholar]
- 32.McClain RM, Rubio F, Dairman W, Koechlin B. 1980. Factors related to increased susceptibility to intestinal lesions with nonsteroidal antiinflammatory agents in the lactating rat. Toxicol Appl Pharmacol 56:383–391 [DOI] [PubMed] [Google Scholar]
- 33.McKellar QA, Delatour P, Lees P. 1994. Stereospecific pharmacodynamics and pharmacokinetics of carprofen in the dog. J Vet Pharmacol Ther 17:447–454 [DOI] [PubMed] [Google Scholar]
- 34.Mealey KL, Matthews NS, Peck KE, Burchfield ML, Bennett BS, Taylor TS. 2004. Pharmacokinetics of R(–) and S(+) carprofen after administration of racemic carprofen in donkeys and horses. Am J Vet Res 65:1479–1482 [DOI] [PubMed] [Google Scholar]
- 35.Meijer MK, Spruijt BM, van Zutphen LF, Baumans V. 2006. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab Anim 40:382–391 [DOI] [PubMed] [Google Scholar]
- 36.Montoya L, Ambros L, Kreil V, Bonafine R, Albarellos G, Hallu R, Soraci A. 2004. A pharmacokinetic comparison of meloxicam and ketoprofen following oral administration to healthy dogs. Vet Res Commun 28:415–428 [DOI] [PubMed] [Google Scholar]
- 37.Mosher RA, Coetzee JF, Cull CA, Gehring R, Kukanich B. 2012. Pharmacokinetics of oral meloxicam in ruminant and preruminant calves. J Vet Pharmacol Ther 35:373–381 [DOI] [PubMed] [Google Scholar]
- 38.Naidoo V, Wolter K, Cromarty AD, Bartels P, Bekker L, McGaw L, Taggart MA, Cuthbert R, Swan GE. 2008. The pharmacokinetics of meloxicam in vultures. J Vet Pharmacol Ther 31:128–134 [DOI] [PubMed] [Google Scholar]
- 39.Ontario Ministry of Agriculture and Food. [Internet]. Animals for Research Act. R.R.O. 1990, Regulation 24 research facilities and supply facilities. [Cited 18 August 2013]. Available at: http://www.elaws.gov.on.ca/html/regs/english/elaws_regs_900024_e.htm.
- 40.Priymenko N, Garnier F, Ferre JP, Delatour P, Toutain PL. 1998. Enantioselectivity of the enterohepatic recycling of carprofen in the dog. Drug Metab Dispos 26:170–176 [PubMed] [Google Scholar]
- 41.Roughan JV, Wright-Williams SL, Flecknell PA. 2009. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim 43:17–26 [DOI] [PubMed] [Google Scholar]
- 42.Schmid J, Busch U, Heinzel G, Bozler G, Kaschke S, Kummer M. 1995. Pharmacokinetics and metabolic pattern after intravenous infusion and oral administration to healthy subjects. Drug Metab Dispos 23:1206–1213 [PubMed] [Google Scholar]
- 43.Schmid J, Busch U, Trummlitz G, Prox A, Kaschke S, Wachsmuth H. 1995. Meloxicam: metabolic profile and biotransformation products in the rat. Xenobiotica 25:1219–1236 [DOI] [PubMed] [Google Scholar]
- 44.Shukla M, Singh G, Sindhura BG, Telang AG, Rao GS, Malik JK. 2007. Comparative plasma pharmacokinetics of meloxicam in sheep and goats following intravenous administration. Comp Biochem Physiol C Toxicol Pharmacol 145:528–532 [DOI] [PubMed] [Google Scholar]
- 45.Sinclair MD, Mealey KL, Matthews NS, Peck KE, Taylor TS, Bennett BS. 2006. Comparative pharmacokinetics of meloxicam in clinically normal horses and donkeys. Am J Vet Res 67:1082–1085 [DOI] [PubMed] [Google Scholar]
- 46.Soraci A, Benoit E, Jaussaud P, Lees P, Delatour P. 1995. Enantioselective glucuronidation and subsequent biliary excretion of carprofen in horses. Am J Vet Res 56:358–361 [PubMed] [Google Scholar]
- 47.Stoltenborg JK, Puglisi CV, Rubio F, Vane FM. 1981. High-performance liquid chromatographic determination of stereoselective disposition of carprofen in humans. J Pharm Sci 70:1207–1212 [DOI] [PubMed] [Google Scholar]
- 48.Taylor PM, Delatour P, Landoni FM, Deal C, Pickett C, Shojaee Aliabadi F, Foot R, Lees P. 1996. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat. Res Vet Sci 60:144–151 [DOI] [PubMed] [Google Scholar]
- 49.Toutain PL, Bousquet-Melou A. 2004. Bioavailability and its assessment. J Vet Pharmacol Ther 27:455–466 [DOI] [PubMed] [Google Scholar]
- 50.Toutain PL, Bousquet-Melou A. 2004. Plasma clearance. J Vet Pharmacol Ther 27:415–425 [DOI] [PubMed] [Google Scholar]
- 51.Toutain PL, Bousquet-Melou A. 2004. Volumes of distribution. J Vet Pharmacol Ther 27:441–453 [DOI] [PubMed] [Google Scholar]
- 52.Toutain PL, Reymond N, Laroute V, Garcia P, Popot MA, Bonnaire Y, Hirsch A, Narbe R. 2004. Pharmacokinetics of meloxicam in plasma and urine of horses. Am J Vet Res 65:1542–1547 [DOI] [PubMed] [Google Scholar]
- 53.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613 [PMC free article] [PubMed] [Google Scholar]
- 54.Turner PV, Pekow C, Vasbinder MA, Brabb T. 2011. Administration of substances to laboratory animals: equipment considerations, vehicle selection, and solute preparation. J Am Assoc Lab Anim Sci 50:614–627 [PMC free article] [PubMed] [Google Scholar]
- 55.Wasfi IA, Al Ali WA, Agha BA, Kamel AM, Al Biriki NA, Al Neaimi KM. 2012. The pharmacokinetics and metabolism of meloxicam in camels after intravenous administration. J Vet Pharmacol Ther 35:155–162 [DOI] [PubMed] [Google Scholar]
- 56.Welsh EM, Baxter P, Nolan AM. 1992. Pharmacokinetics of carprofen administered intravenously to sheep. Res Vet Sci 53:264–266 [DOI] [PubMed] [Google Scholar]
- 57.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mouse. Pain 130:108–118 [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y, Chen XY, Li SM, Wei XY, Yao HM, Zhong DF. 2009. Pharmacokinetic studies of meloxicam following oral and transdermal administration in beagle dogs. Acta Pharmacol Sin 30:1060–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]