Abstract
Cannulation of the common carotid artery for chronic, continuous radiotelemetric recording of aortic hemodynamic properties in mice is a highly invasive recovery surgery. Radiotelemetric recording, by its continuous nature, gives the most accurate measurements of hemodynamic variables in experimental animals, and is widely used in the study of cardiovascular diseases including hypertension. The American Heart Association has recommended data acquisition by radiotelemetric recording but did not provide guidelines regarding postoperative analgesic support. We assessed hemodynamic parameters, locomotor activity, food intake, and weight loss in radiotransmitter-implanted CD1 female mice receiving analgesic support during the first 48 h after surgery. The efficacy of analgesic support from the NSAID meloxicam was compared with that of the widely used opioid agonist buprenorphine and the related compound, tramadol. Meloxicam-treated mice recovered lost body weight more rapidly than did tramadol- or buprenorphine-treated mice. Furthermore, meloxicam-treated mice maintained circadian rhythm after surgery and had tighter regulation of mean arterial pressure than did tramadol- or buprenorphine-treated mice. Meloxicam was also superior with regard to food intake, locomotor activity, and limiting variance in hemodynamic parameters. This study indicates that when compared with buprenorphine and tramadol, meloxicam should be the postoperative analgesic of choice for radiotelemeter implantation in mice.
Abbreviation: HR, heart rate; MAP, mean arterial pressure
Measurement of arterial blood pressure in experimental animals is tremendously important for the study of numerous diseases, including hypertension. Currently, the most accurate and highly validated method for measuring arterial pressure in experimental animals is radiotelemetry.32 Implantation of the devices requires a skilled surgeon, and rodents need at least 7 d to recover fully after surgery.22 The immense stress placed on animals by radiotelemeter implantation surgery has prompted numerous evaluations of novel implantation procedures.9,22,29,30,34,42 In addition, limited evidence is available regarding which postoperative analgesic provides the greatest benefit to recovering animals. When describing the use of radiotelemetry, American Heart Association guidelines covering the measurement of blood pressure in experimental animals make no mention of analgesia.32 Buprenorphine is currently the most commonly used postoperative analgesic after telemeter implantation surgery.9,19,30 However, its efficacy in providing postoperative pain management in the absence of adverse side effects has been questioned.3,4,28,46
Buprenorphine is a centrally acting partial μ-opioid receptor agonist and a δ- and κ-receptor antagonist that exhibits approximately 75 to 100 times the antinociceptive effect of morphine.46 Because buprenorphine is an opioid analgesic, its use is legislatively controlled in most jurisdictions. Tramadol has similar effects to buprenorphine, also acting as a μ-receptor agonist.35 Tramadol has a lower affinity for the μ receptor than does buprenorphine and therefore displays a significantly shorter half-life.35 In addition to being a μ-receptor agonist, tramadol's analgesic activity is mediated through inhibiting norepinephrine and serotonin reuptake, thus blocking nociceptive impulses at the spinal level.23 Meloxicam, another commonly used analgesic, is a NSAID and selective inhibitor of cyclooxygenase 2 inhibitor.18 Meloxicam's mechanism of analgesia is via inhibition of synthesis of prostaglandin H2, a precursor of mediators that elicit pain and inflammation.18
The postoperative effects of analgesics involve more than just pain relief. Commonly used analgesics are known to modify locomotor activity,28 food consumption,3 fluctuations in body weight,5 and hemodynamic factors.4,28 Effective analgesia minimizes changes to animal behavior over this array of experimental paradigms as it reduces pain and distress. The purpose of the current study was to compare the effects of standard doses of commonly used analgesics in mice recovering from cannulation of the common carotid artery for placement of a radiotelemetric catheter tip into the aortic arch. This surgery is accompanied by subcutaneous placement of an approximately 1 cm × 1 cm × 1 cm radiotelemeter in the lateral flank and is classed as a chronic, recovery intervention category D animal care protocol in Canada.8 We found that, compared with tramadol and buprenorphine, meloxicam provided superior postoperative recovery, supporting full recovery of mice in less time and with fewer disruptions to normal homeostasis.
Materials and Methods
Animals.
All experiments were performed in the Animal Care Facilities at Queen's University under protocols approved by the Queen's University Animal Care Committee. Female CD1 mice (n = 21; age, 10 to 12 wk) were purchased from Charles River (St Constant, Quebec, Canada) and housed under conventional husbandry. The mice had ad libitum access to food and water and were maintained on a 12:12-h light:dark cycle at an ambient temperature of 23 °C and 15% to 25% relative humidity. Mice were tested quarterly and found to be serologically negative for the following agents: Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, murine norovirus, Theiler murine encephalomyelitis virus, reovirus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, mousepox (ectromelia) virus, mouse adenovirus, mouse cytomegalovirus, mouse pneumonitis virus (K virus), polyoma virus, Hantaan virus, mouse thymic virus, B. bronchiseptica, cilia-associated respiratory virus, Corynebacterium rodentium, C. kutscheri, Hepatobacter bilis, H. hepaticus, Helicobacter spp., Klebsiella pneumoniae, Mycoplasma pulmonis, Pasteurella multocida, P. pneumotropica, Salmonella spp., Streptococcus moniliformis, S. pneumoniae, β-Streptococcus spp., and Clostridium piliforme. In addition, mice were free of endoparasites and ectoparasites.
Telemeter implantation surgery.
Radiotransmitters (model TA11PA-C10, Data Sciences International, St Paul, MN) were implanted via the left common carotid artery, as reported previously.7 Briefly, mice were anesthetized with isoflurane. The cervical ventral midline was incised (2 cm), and the submandibular glands were separated by using sterile cotton swabs. The left common carotid artery was visualized, retracted, and temporarily occluded with a microvessel clamp. The artery was punctured with a 26-gauge needle, the catheter tip of the transmitter was advanced to the aortic arch by using cannulation forceps, and the catheter was sutured in place. A subcutaneous pocket was excavated over the right flank, the transmitter body was inserted into this pocket, and the incision was closed (4-0 polyglactin 910; Vicryl, Ethicon, Somerville, NJ). All surgeries were conducted between 0900 and 1200.
Postoperative treatment and recovery.
Mice were assigned randomly to receive buprenorphine, tramadol, or meloxicam immediately after induction with isoflurane, ensuring the drug's effect on recovery. Mice were individually housed for recovery and started on continuous, 24-h data collection. The buprenorphine group (n = 8) was treated twice daily at 0900 and 1600 with buprenorphine (0.05 mg/kg SC; Temgesic, Schering–Plough, Pointe Claire, Quebec, Canada); the tramadol group (n = 8) was treated once daily (20 mg/kg SC; Chiron Compounding, Guelph, Ontario, Canada); the meloxicam group (n = 5) was treated with 2 mg/kg meloxicam (Metacam, Boehringer Ingelheim Vetmedica, St Louis, MO) after induction of anesthesia and then once daily with 1 mg/kg. All treatments were administered for 48 h after surgery. Dosages for analgesic agents were determined based on previously recommended values.14,26,33,45 Food consumption and body weights were recorded daily for 7 d after surgery. In accordance with the animal care guidelines at Queen's University, mice that lost more than 15% body weight after surgery were euthanized and excluded from the study. At 10 d after surgery, the mice entered other studies addressing gestational regulation of blood pressure.2 An additional 3 mice were randomized to receive the meloxicam treatment, but these animals experienced problems with signal transmission from the radiotransmitter unit and did not provide complete data sets for the recovery period. These 3 incomplete recordings were excluded, and only the remaining 5 complete data sets were included in final analysis. No mice died as a direct result of the surgery, nor were any removed from the study due to excessive (greater than 15%) weight loss.
Data acquisition.
Immediately after surgery, continuous, 24-h data collection began (version 4.1, Dataquest ART Acquisition System, Data Sciences International). Collected parameters included mean arterial pressure (MAP), systolic blood pressure, diastolic blood pressure, heart rate (HR), pulse pressure, and activity. Data were acquired from transmitters for 30-s intervals every 4 min, yielding 360 measurements for every 24-h period. To minimize the effects of light-dark–cycle changes, daytime telemetry data presented in the current study represent means of all data points collected between the hours of 1000 and 1600, and nighttime data represent the points collected between the hours of 2200 and 0400 (90 total measurements for each day and night). This time period corresponds to the 6-h interval during the middle of each lighting period in the Animal Care Facilities at Queen's University, which cycles from dark to light at 0700 and from light to dark at 1900.
Statistics.
Recorded body weights were corrected for the weight of the transmitter (1.4 g) and normalized to preoperative baseline values, which were measured immediately prior to surgery. Food consumption, locomotor activity, and hemodynamic parameters were normalized to postoperative day 7 values, which were consistent with values obtained 2 wk after surgery.2 All data were analyzed within groups by repeated-measures one-way ANOVA with Dunnett posthoc test for postoperative day and 2-way ANOVA with Bonferroni posthoc test for postoperative analgesic treatment (Prism 5.00, GraphPad, San Diego, CA). Mean variation associated with each parameter was calculated as the absolute area under the curve within a given period. Data are presented as mean ± SEM, with a P value of less than 0.05 considered statistically significant.
Results
Body weight and food consumption.
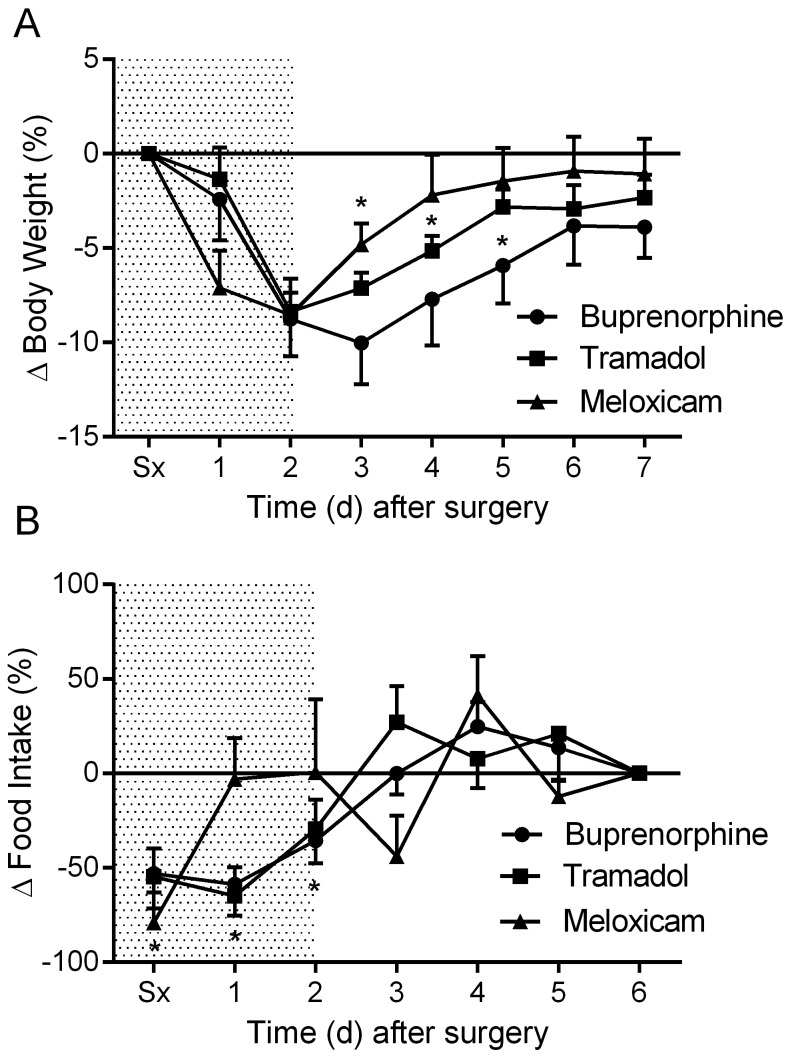
All groups of mice exhibited an approximate 8.5% (8.4% to 8.8%) drop in body weight within the first 48 h after surgical implantation of transmitters (Figure 1 A). The body weights of the meloxicam-treated mice were significantly (P < 0.05) decreased from postoperative day 1 to day 3; body weight recovery was achieved on postoperative day 4. The tramadol- and buprenorphine-treated mice had significantly (P < 0.05) decreased body weights from postoperative day 2 to days 4 and 5, respectively (Figure 1 A). The tramadol-treated mice recovered body weight loss at postoperative day 5, whereas the buprenorphine-treated mice regained baseline body weight at day 6.
Figure 1.
The effects of analgesics on postoperative changes in (A) body weight and (B) food intake. Shaded area indicates postoperative treatment period. Data are expressed as mean ± SEM. *, P < 0.05 compared with baseline value; Sx, day of surgery.
The losses in body weight were linked with decreased food consumption. Tramadol- and buprenorphine-treated mice consumed significantly (P < 0.05) less food for the first 3 postoperative days, whereas the meloxicam-treated mice displayed decreased (P < 0.05) food consumption for only the first day after surgery, with restoration of baseline food consumption on postoperative day 6 (Figure 1 B).
MAP, HR, and locomotor activity.
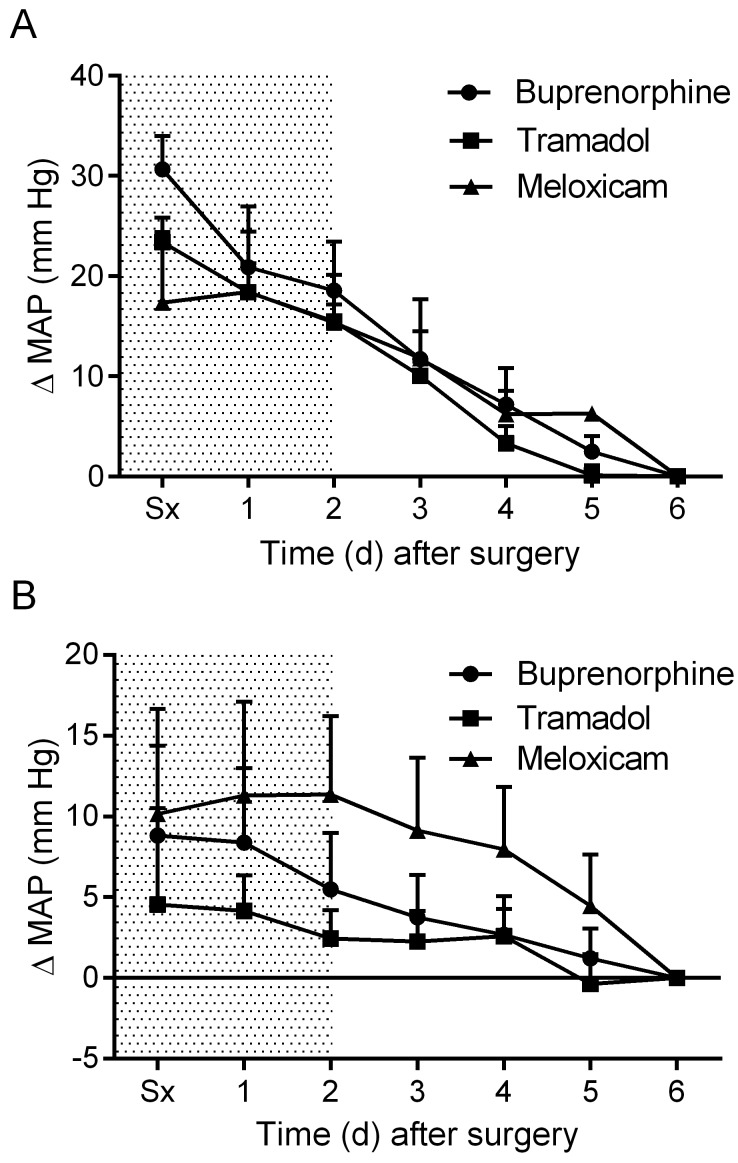
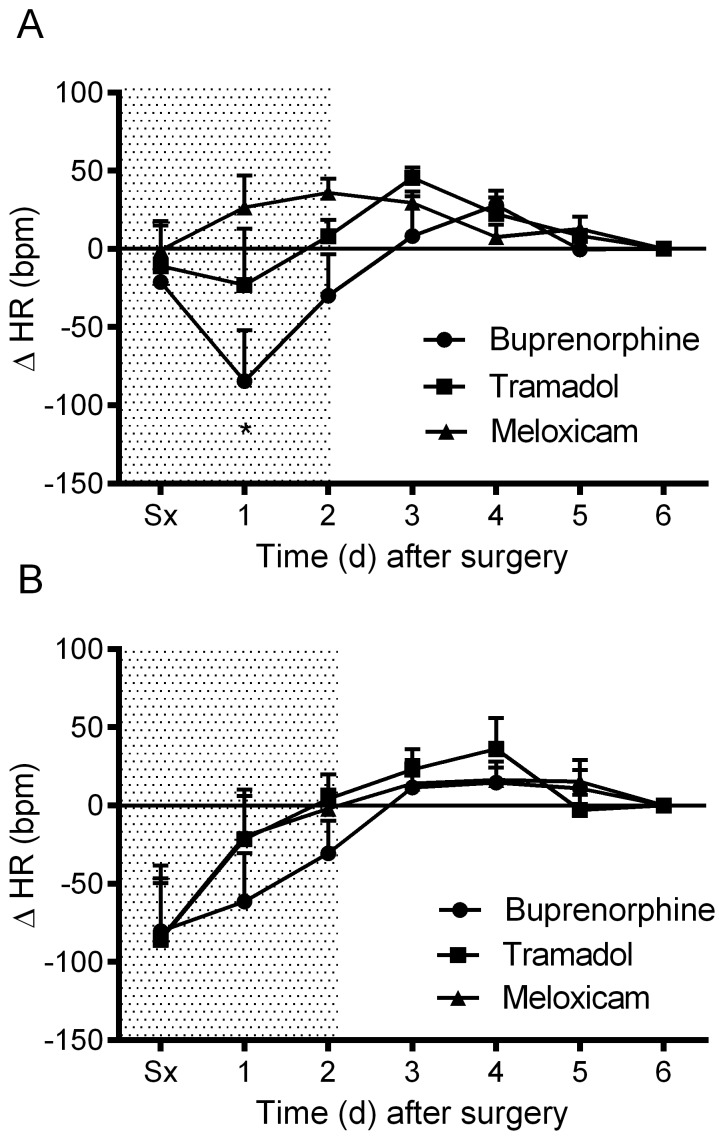
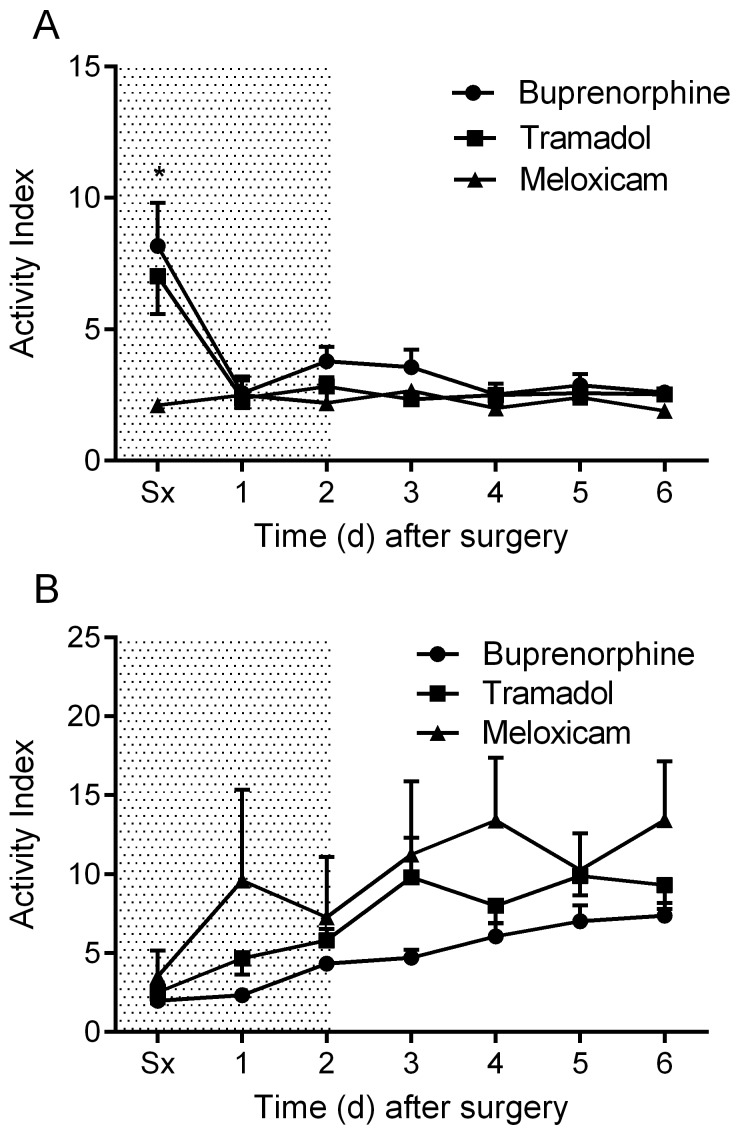
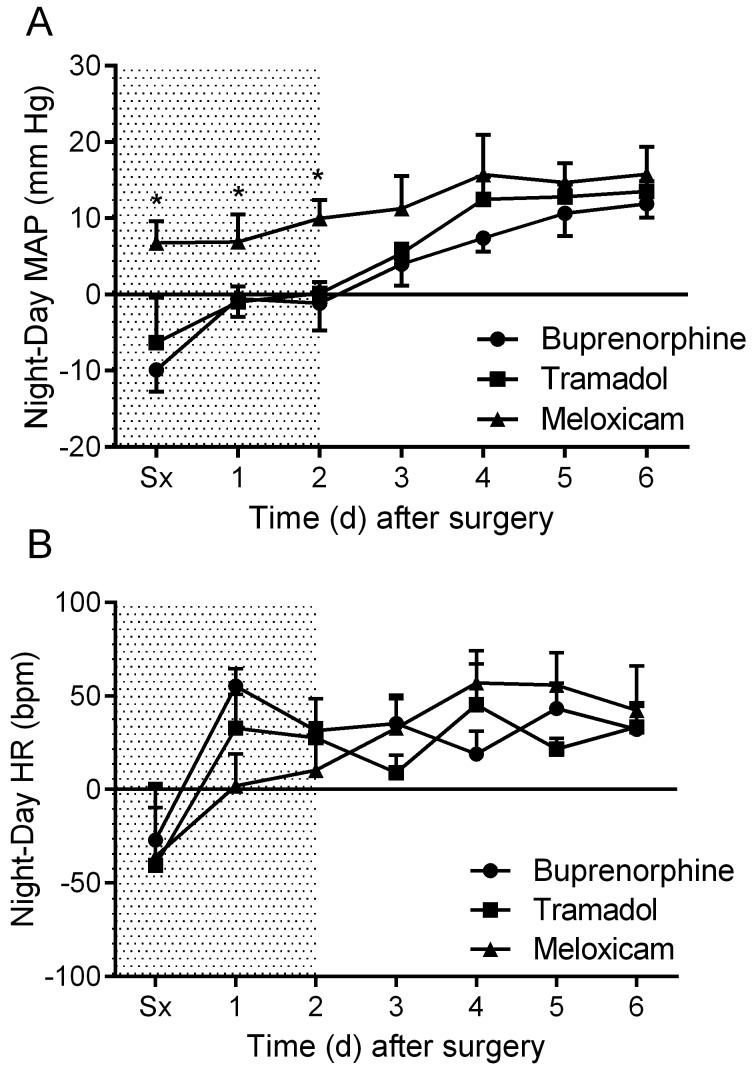
Daytime MAP was elevated in all treatment groups after surgery and declined gradually during the postoperative period (Figure 2 A). Nighttime MAP was similarly elevated in all treatment groups immediately after surgery (Figure 2 B). No significant differences in MAP were observed between groups. The meloxicam- and tramadol-treated mice did not display a daytime change in HR throughout the recovery period, whereas the buprenorphine-treated mice exhibited a significant (P < 0.05) drop in daytime HR on postoperative day 1 (Figure 3 A). Nighttime HR was significantly (P < 0.05) lower than baseline in all groups on the surgical day and remained low in the buprenorphine-treated group into postoperative day 1. No differences in nighttime HR were noted between groups (Figure 3 B). Daytime locomotor activity was significantly (P < 0.05) elevated immediately after surgery in the tramadol- and buprenorphine-treated mice; however, the meloxicam-treated mice did not display a daytime change in activity throughout recovery (Figure 4 A). Nighttime locomotor activity on the day of surgery was blunted in all groups. No significant differences in nighttime locomotor activity occurred between groups (Figure 4 B).
Figure 2.
The effects of analgesics on postoperative changes in mean arterial pressure (MAP) during the (A) day and (B) night. Shaded area indicates postoperative treatment period. Data are expressed as mean ± SEM. Sx, day of surgery.
Figure 3.
The effects of analgesics on postoperative changes in heart rate (HR) during the (A) day and (B) night. Shaded area indicates postoperative treatment period. Data are expressed as mean ± SEM. *, P < 0.05 compared with baseline value; Sx, day of surgery.
Figure 4.
The effects of analgesics on postoperative changes in locomotor activity during the (A) day and (B) night. Shaded area indicates postoperative treatment period. Data are expressed as mean ± SEM. *, P < 0.05 compared with value for meloxicam group; Sx, day of surgery.
Circadian rhythm.
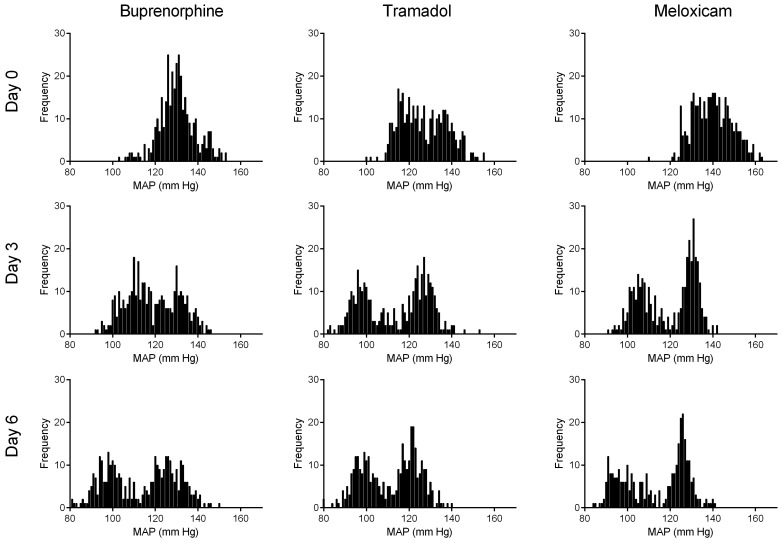
Mice treated with either tramadol or buprenorphine displayed a reversal of normal MAP circadian rhythm on the day of surgery, with higher MAP during daytime than nighttime (Figure 5 A). In contrast, mice treated with meloxicam displayed only a slight reduction in the overall mean difference between night and day MAP values and maintained greater nighttime MAP compared with daytime for the duration of the study (P < 0.05; Figure 5 A). In all treatment groups, mice displayed a reversal of HR circadian rhythm the day of surgery and recovered similarly thereafter (Figure 5 B). MAP normally shows a biphasic daily distribution,36 which was not present during the first 24 h postoperatively for any treatment group (Figure 6). Buprenorphine showed the greatest loss of this pattern (that is, narrowest range). Biphasic patterns in MAP variability for all treatment groups had returned by postoperative day 3. On postoperative day 3, mice treated with meloxicam displayed tighter regulation of MAP than did mice treated with buprenorphine or tramadol (Figure 6). By postoperative day 6, the distribution of MAP values in buprenorphine- and tramadol-treated mice had improved but still were wider (that is, less tightly regulated) than were those in meloxicam-treated mice (Figure 6).
Figure 5.
The effects of analgesics on postoperative changes to circadian rhythm in regard to (A) mean arterial pressure (MAP) and (B) heart rate (HR). Data are expressed as mean ± SEM. *, P < 0.05 compared with values for buprenorphine and tramadol groups; Sx, day of surgery.
Figure 6.
24-h frequency distributions of mean arterial pressure (MAP) on days 0, 3, and 6 after telemeter implantation. Data shown are from a representative animal in each treatment group.
Discussion
The present study found a clear superiority of meloxicam over buprenorphine or tramadol as a postoperative analgesia after radiotelemeter implantation surgery in mice. Only commonly recommended dosages were compared,14,26,33,45 and the key finding was that the recovery of weight loss was delayed after treatment with buprenorphine or tramadol compared with meloxicam. This finding is consistent with previous data from our laboratory that compared postoperative buprenorphine and meloxicam in rats.4 Other colleagues have reported that postoperative buprenorphine significantly reduced growth rates in young rats.5 Moreover, growth suppression after buprenorphine administration occurred in rats that had not undergone a surgical procedure.5 Our present study identified decreased food intake after surgery in the tramadol- and buprenorphine-treated mice, whereas food intake remained closer to presurgical values in mice treated with meloxicam. It has been demonstrated previously that nonselective opioid receptor antagonists can suppress appetite.1 Although both buprenorphine and tramadol achieve their analgesic effects through μ-opioid receptor agonism, each drug also has antagonistic effects on the δ- and κ-receptor subtypes.35,46 Meloxicam, in contrast, attenuates zymosan-induced anorexic responses39 and displays strong gastrointestinal tolerability.11,17,37,41,44,47
In addition to their apparent disruptive effects on normal eating behavior, buprenorphine and tramadol affected hemodynamic parameters. Mice treated with buprenorphine exhibited the greatest increase in MAP immediately after surgery, most likely due to direct effects causing acute increases in body temperature, heart rate, blood pressure, and locomotor activity, as in rats.27,28 Interestingly, buprenorphine treated mice did not exhibit elevated MAP or activity levels during nighttime. In mice, buprenorphine displays a relatively short elimination half-life (approximately 2.9 h),49 which would explain its effects on hemodynamic factors only during the day. Interestingly, daytime locomotor activity during the first postoperative day was increased greatly in the buprenorphine- and tramadol-treated groups but remained at baseline for meloxicam-treated mice. This difference may represent an acute action of the opioid analgesics, as demonstrated previously in rats.28 Because mice are nocturnal, it might have been useful to administer opioid analgesia during the night period instead.
An important and novel finding of the current study was that the administration of buprenorphine and tramadol as postoperative analgesia reversed normal circadian rhythm, whereas meloxicam analgesia maintained it. Major surgery can disrupt normal circadian rhythm,21 and these disturbances negatively correlate with optimal postoperative recovery.20 In addition, disruption of normal sleep patterns interfere with postoperative recovery and mediate an increase in postoperative pain.12 The high locomotor activity levels during the day period immediately after surgery in the mice that received buprenorphine and tramadol recorded suggest that these animals had disrupted sleep patterns, which could have affected their overall recovery.
Postoperative analgesia conferred by NSAID may provide additional benefits over the use of traditional opioid analgesia. Buprenorphine and tramadol are known to cause significant hyperalgesia,40,48 respiratory depression,13,16,43 and acute hyperactivity and hyperthermia.27,28 Conversely, meloxicam has little or no effect on cardiovascular and respiratory parameters,18 renal function10,24 or gastrointestinal activity.37,44 Furthermore, because meloxicam achieves its analgesic effects without antagonizing nociceptive receptors, the risk of sensitization is minimal.18 The longer duration of action of meloxicam (t1/2 = 6.4 h)6 as compared with that of buprenorphine also provides animal and personnel resource benefits, in that meloxicam requires administration only once daily, whereas buprenorphine requires twice-daily administration to achieve continuous analgesic effects. In addition, buprenorphine and tramadol, as opioid narcotics, are designated as controlled substances in many jurisdictions; meloxicam, in contrast, is available for use without the need for special permits or exemptions.
Compared with classic NSAID, such as ketoprofen, meloxicam appears to provide similar analgesic effects15,38 and displays strong gastrointestinal tolerability11 and limited disruption to renal function.10,17,25 However, most head-to-head comparisons of meloxicam and classic NSAID have been done in dogs, cats, and humans and therefore would not be directly applicable to the present study in mice. In addition, meloxicam exhibits more COX2 selectivity than do classic NSAID; therefore, extending the favorable outcomes mediated by meloxicam to classic NSAID may be ill-advised. A direct comparison of classic NSAID with meloxicam in mice is warranted, because the use of classic NSAID as postoperative analgesia may provide an additional cost benefit over the use of meloxicam.
Postoperative analgesia for laboratory animals is not only morally imperative for investigators but also promotes the return of normal animal function and hastens the time of onset for quality data collection.27,28 The operating cost associated with radiotelemetric monitoring of hemodynamic parameters is substantial, making the acquisition of accurate data in telemetric experiments of central importance. Appropriate and effective analgesia after surgical implantation of devices can provide marked benefit to the cost-effectiveness of these procedures by reducing the time needed for recovery27 and by improving postoperative survival.31
Radiotelemetry is recognized as the most accurate and valid method for measuring hemodynamic parameters in laboratory rodents. However, the surgical implantation of these devices is highly invasive and potentially disruptive to the animal's health and wellbeing. Here, the postoperative recovery of CD1 mice after radiotelemeter implantation into the aortic arch was monitored to compare the efficacy of standard doses of 3 agents in supporting cardiovascular and behavioral recovery. The study demonstrated the clear superiority of meloxicam over the traditional opioid analgesics buprenorphine and tramadol. Meloxicam provided superior postoperative benefits in regard to body weight, food consumption, hemodynamic factors, and circadian rhythm. Furthermore, this study demonstrated the undesirable side effects of postoperative opioid analgesic administration on data that are collected before mice complete an adequate recovery period. These drawbacks included higher daytime activity and wider variability across data sets. Selection of postoperative analgesia must be considered among the variables influencing radiotelemetric data collection in mice. Meloxicam produces strong analgesic effects45 and causes the least disruption to postoperative recovery and hemodynamic parameters. Therefore, we recommend meloxicam over buprenorphine and tramadol as the postoperative analgesia of choice after surgical telemeter implantation in mice.
Acknowledgments
This research was supported by awards from the Natural Sciences and Engineering Research Council of Canada (to BAC) and the Canadian Foundation for Innovation (to MAA and BAC). MTR is supported by awards from the Ontario Graduate Scholarship and Frederick Banting and Charles Best Canada Graduate Scholarship. BAC is supported by the Canada Research Chairs program.
References
- 1.Barbano MF, Cador M. 2006. Differential regulation of the consummatory, motivational, and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology 31:1371–1381 [DOI] [PubMed] [Google Scholar]
- 2.Barrette VF, Adams MA, Croy BA. 2012. Endometrial decidualization does not trigger the blood pressure decline of normal early pregnancy in mice. Biol Reprod 86:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaha MD, Leon LR. 2008. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci 47:8–19 [PMC free article] [PubMed] [Google Scholar]
- 4.Bourque SL, Adams MA, Nakatsu K, Winterborn A. 2010. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49:617–622 [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan MP, Sinusas AJ, Horvath TL, Collins JG, Harding MJ. 2009. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim (NY) 38:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch U, Schmid J, Heinzel G, Schmaus H, Baierl J, Huber C, Roth W. 1998. Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos 26:576–584 [PubMed] [Google Scholar]
- 7.Butz GM, Davisson RL. 2001. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5:89–97 [DOI] [PubMed] [Google Scholar]
- 8.Canadian Council on Animal Care [Internet]. 1991. CCAC policy statement on: categories of invasiveness in animal experiments. [Cited August 2013]. Available at: http://www.ccac.ca/en_/standards/policies/policy-categories_of_invasiveness
- 9.Chappell MG, Koeller CA, Hall SI. 2011. Differences in postsurgical recovery of CF1 mice after intraperitoneal implantation of radiotelemetry devices through a midline or flank surgical approach. J Am Assoc Lab Anim Sci 50:227–237 [PMC free article] [PubMed] [Google Scholar]
- 10.Crandell DE, Mathews KA, Dyson DH. 2004. Effect of meloxicam and carprofen on renal function when administered to healthy dogs prior to anesthesia and painful stimulation. Am J Vet Res 65:1384–1390 [DOI] [PubMed] [Google Scholar]
- 11.Craven M, Chandler ML, Steiner JM, Farhadi A, Welsh E, Pratschke K, Shaw DJ, Williams DA. 2007. Acute effects of carprofen and meloxicam on canine gastrointestinal permeability and mucosal absorptive capacity. J Vet Intern Med 21:917–923 [DOI] [PubMed] [Google Scholar]
- 12.Cremeans-Smith JK, Millington K, Sledjeski E, Greene K, Delahanty DL. 2006. Sleep disruptions mediate the relationship between early postoperative pain and later functioning following total knee replacement surgery. J Behav Med 29:215–222 [DOI] [PubMed] [Google Scholar]
- 13.Dahan A, Aarts L, Smith TW. 2010. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112:226–238 [DOI] [PubMed] [Google Scholar]
- 14.Dal D, Salman M, Salman A, Iskit A, Aypar U. 2006. The involvement of nitric oxide on the analgesic effect of tramadol. Eur J Anaesthesiol 23:175–177 [DOI] [PubMed] [Google Scholar]
- 15.Deneuche AJ, Dufayet C, Goby L, Fayolle P, Desbois C. 2004. Analgesic comparison of meloxicam or ketoprofen for orthopedic surgery in dogs. Vet Surg 33:650–660 [DOI] [PubMed] [Google Scholar]
- 16.Etches RC, Sandler AN, Daley MD. 1989. Respiratory depression and spinal opioids. Can J Anaesth 36:165–185 [DOI] [PubMed] [Google Scholar]
- 17.Forsyth SF, Guilford WG, Haslett SJ, Godfrey J. 1998. Endoscopy of the gastroduodenal mucosa after carprofen, meloxicam and ketoprofen administration in dogs. J Small Anim Pract 39:421–424 [DOI] [PubMed] [Google Scholar]
- 18.Gates BJ, Nguyen TT, Setter SM, Davies NM. 2005. Meloxicam: a reappraisal of pharmacokinetics, efficacy, and safety. Expert Opin Pharmacother 6:2117–2140 [DOI] [PubMed] [Google Scholar]
- 19.Goecke JC, Awad H, Lawson JC, Boivin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44 [PubMed] [Google Scholar]
- 20.Gogenur I, Bisgaard T, Burgdorf S, van Someren E, Rosenberg J. 2009. Disturbances in the circadian pattern of activity and sleep after laparoscopic versus open abdominal surgery. Surg Endosc 23:1026–1031 [DOI] [PubMed] [Google Scholar]
- 21.Gogenur I, Rosenberg-Adamsen S, Kiil C, Kjaersgaard M, Kehlet H, Rosenberg J. 2001. Laparoscopic cholecystectomy causes less sleep disturbance than open abdominal surgery. Surg Endosc 15:1452–1455 [DOI] [PubMed] [Google Scholar]
- 22.Greene AN, Clapp SL, Alper RH. 2007. Timecourse of recovery after surgical intraperitoneal implantation of radiotelemetry transmitters in rats. J Pharmacol Toxicol Methods 56:218–222 [DOI] [PubMed] [Google Scholar]
- 23.Grond S, Sablotzki A. 2004. Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923 [DOI] [PubMed] [Google Scholar]
- 24.Harirforoosh S, Aghazadeh-Habashi A, Jamali F. 2006. Extent of renal effect of cyclooxygenase-2-selective inhibitors is pharmacokinetics-dependent. Clin Exp Pharmacol Physiol 33:917–924 [DOI] [PubMed] [Google Scholar]
- 25.Harirforoosh S, Jamali F. 2008. Effect of inflammation on kidney function and pharmacokinetics of COX2 selective nonsteroidal antiinflammatory drugs rofecoxib and meloxicam. J Appl Toxicol 28:829–838 [DOI] [PubMed] [Google Scholar]
- 26.Hawk CT, Leary SL, Morris TH. 2005. Formulary for laboratory animals. Ames (IA): Blackwell Publishing. [Google Scholar]
- 27.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 28.Ilback NG, Siller M, Stalhandske T. 2008. Effects of buprenorphine on body temperature, locomotor activity and cardiovascular function when assessed by telemetric monitoring in rats. Lab Anim 42:149–160 [DOI] [PubMed] [Google Scholar]
- 29.Johnston NA, Bosgraaf C, Cox L, Reichensperger J, Verhulst S, Patten C, Jr, Toth LA. 2007. Strategies for refinement of abdominal device implantation in mice: strain, carboxymethylcellulose, thermal support, and atipamezole. J Am Assoc Lab Anim Sci 46:46–53 [PubMed] [Google Scholar]
- 30.Kaidi S, Brutel F, Van Deun F, Kramer K, Remie R, Dewe W, Remusat P, Delaunois A, Depelchin O. 2007. Comparison of 2 methods (left carotid artery and abdominal aorta) for surgical implantation of radiotelemetry devices in CD1 mice. Lab Anim 41:388–402 [DOI] [PubMed] [Google Scholar]
- 31.Krueger KL, Fujiwara Y. 2008. The use of buprenorphine as an analgesic after rodent embryo transfer. Lab Anim (NY) 37:87–90 [DOI] [PubMed] [Google Scholar]
- 32.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. 2005. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals. A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arterioscler Thromb Vasc Biol 25:e22–e33 [DOI] [PubMed] [Google Scholar]
- 33.Leach MC, Klaus K, Miller A, Scotto di Perrotolo M, Sotocinal S, Flecknell P. 2012. The assessment of post-vasectomy pain in mice using behaviour and the mouse grimace scale. PLoS ONE 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leon LR, Walker LD, DuBose DA, Stephenson LA. 2004. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am J Physiol Regul Integr Comp Physiol 286:R967–R974 [DOI] [PubMed] [Google Scholar]
- 35.Leppert W. 2009. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep 61:978–992 [DOI] [PubMed] [Google Scholar]
- 36.Li P, Sur SH, Mistlberger RE, Morris M.1999. Circadian blood pressure and heart rate rhythms in mice. Am J Physiol 276:R500–R504. [DOI] [PubMed]
- 37.Luna SP, Basilio AC, Steagall PV, Machado LP, Moutinho FQ, Takahira RK, Brandao CV. 2007. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res 68:258–264 [DOI] [PubMed] [Google Scholar]
- 38.Morton CM, Grant D, Johnston L, Letellier IM, Narbe R. 2011. Clinical evaluation of meloxicam versus ketoprofen in cats suffering from painful acute locomotor disorders. J Feline Med Surg 13:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naoi K, Kogure S, Saito M, Hamazaki T, Watanabe S. 2006. Differential effects of selective cyclooxygenase (COX) 1 and COX2 inhibitors on anorexic response and prostaglandin generation in various tissues induced by zymosan. Biol Pharm Bull 29:1319–1324 [DOI] [PubMed] [Google Scholar]
- 40.Ramasubbu C, Gupta A. 2011. Pharmacological treatment of opioid-induced hyperalgesia: a review of the evidence. J Pain Palliat Care Pharmacother 25:219–230 [DOI] [PubMed] [Google Scholar]
- 41.Schoenfeld P. 1999. Gastrointestinal safety profile of meloxicam: a meta-analysis and systematic review of randomized controlled trials. Am J Med 107:48S–54S [DOI] [PubMed] [Google Scholar]
- 42.Schuler B, Rettich A, Vogel J, Gassmann M, Arras M. 2009. Optimized surgical techniques and postoperative care improve survival rates and permit accurate telemetric recording in exercising mice. BMC Vet Res 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafford HL, Schadt JC. 2008. Respiratory and cardiovascular effects of buprenorphine in conscious rabbits. Vet Anaesth Analg 35:326–332 [DOI] [PubMed] [Google Scholar]
- 44.Singh G, Lanes S, Triadafilopoulos G. 2004. Risk of serious upper gastrointestinal and cardiovascular thromboembolic complications with meloxicam. Am J Med 117:100–106 [DOI] [PubMed] [Google Scholar]
- 45.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 46.Vadivelu N, Anwar M. 2010. Buprenorphine in postoperative pain management. Anesthesiol Clin 28:601–609 [DOI] [PubMed] [Google Scholar]
- 47.Villegas I, Alarcon de la Lastra C, La Casa C, Motilva V, Martin MJ. 2001. Effects of food intake and oxidative stress on intestinal lesions caused by meloxicam and piroxicam in rats. Eur J Pharmacol 414:79–86 [DOI] [PubMed] [Google Scholar]
- 48.Wala EP, Holtman JR., Jr 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651:89–95 [DOI] [PubMed] [Google Scholar]
- 49.Yu S, Zhang X, Sun Y, Peng Y, Johnson J, Mandrell T, Shukla AJ, Laizure SC. 2006. Pharmacokinetics of buprenorphine after intravenous administration in the mouse. J Am Assoc Lab Anim Sci 45:12–16 [PubMed] [Google Scholar]