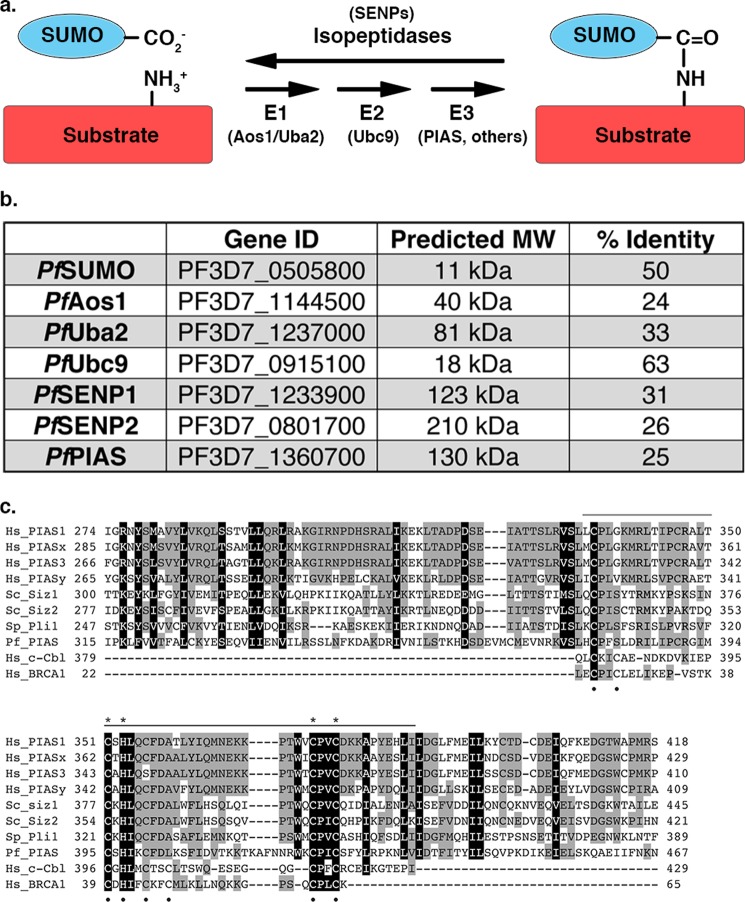
FIGURE 1.
The sumoylation pathway is conserved in P. falciparum. a, SUMO is conjugated to proteins through an enzymatic cascade involving E1-activating and E2-conjugating enzymes and E3 ligases. Deconjugation is catalyzed by isopeptidases. b, enzymes of the P. falciparum sumoylation pathway identified by sequence similarity searches (% identity to human proteins is indicated). Gene ID and predicted molecular weights (MW) were obtained from PlasmoDB. c, amino acid sequence alignment of SP-RING domains from human (Hs), S. cerevisiae (Sc), Schizosaccharomyces pombe (Sp), and Plasmodium falciparum (Pf) SUMO E3 ligases, as well as RING domains from c-Cbl and BRCA1. Sequence conservation is highlighted in gray and black. The SP-RING domain is indicated by the top bar and Zn2+ coordinating cysteine and histidine residues are indicated by black dots. Cysteine and histidine residues unique to SP-RING domains are indicated with asterisks.