FIGURE 3.
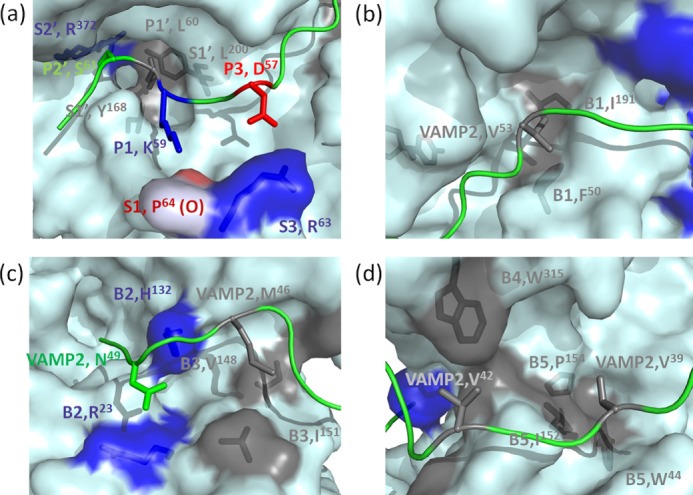
Specific recognition of VAMP-2 by LC/D pockets. Shown are surface representations of the recognition of different P sites of VAMP-2 by the S pockets and recognition of VAMP-2-binding sites by the B1–B5 binding sites of LC/D. Negatively charged residues are shown in red, positively charged residues are shown in blue, hydrophobic residues are shown in gray, and polar residues are shown in green. a, recognition of the P sites of VAMP-2 by the active site pockets of LC/D. The P2′ site (Ser61) of VAMP-2 is recognized by the S2′ pocket (Arg372). The P1′ site (Leu60) of VAMP-2 is recognized by the S1′ pocket (Tyr168 and Leu200) of LC/D. The P1 site (Lys59) of VAMP-2 interacts with the oxygen atom of Pro64 of LC/D. The P3 site (Asp57) of VAMP-2 is recognized by the S3 pocket (Arg63) of LC/D. b, recognition of Val53 by the B1 binding site (Phe50 and Ile191) of LC/D. c, recognition of Asn49 of VAMP-2 by the B2 binding site (Arg23 and His132) of LC/D and recognition of Met46 of VAMP-2 by the B3 binding site (Val148 and Ile151) of LC/D. d, recognition of Val42 by the B4 binding site (Trp315) of LC/D and recognition of Val39 by the B5 binding site (Trp44, Ile152, and Pro154) of LC/D.
