FIGURE 8.
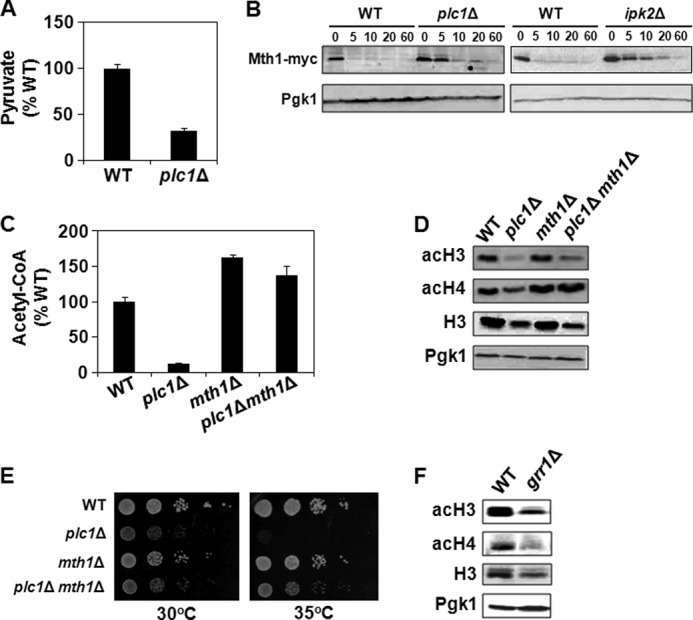
Increased stability of Mth1p is responsible for the decreased level of acetyl-CoA and histone hypoacetylation in plc1Δ cells. A, plc1Δ cells have low intracellular levels of pyruvate. Cells were grown in YPD medium, lysed, and pyruvate was assayed with colorimetric assay kit. The experiment was repeated three times, and the results are shown as mean ± S.D. B, samples from wild-type, plc1Δ, and ipk2Δ cells expressing Mth1-myc were grown in YP medium containing 2% galactose to an A600 = 0.8. Glucose was subsequently added to 4% and samples were taken just before addition of glucose and at the times indicated after the addition of glucose. Cell extracts were analyzed by Western blotting with anti-myc antibodies. Even loading of protein samples was confirmed with anti-Pgk1p antibody. The experiment was performed three times, and representative results are shown. C, low intracellular level of acetyl-CoA in plc1Δ cells is partially suppressed by the mth1Δ mutation. The indicated strains were grown in YPD medium to an A600 = 0.8. The cells were harvested by centrifugation, lysed with glass beads in perchloric acid, and acetyl-CoA was determined in neutralized lysates. The experiment was repeated three times, and the results are shown as mean ± S.D. 100% wild-type levels of acetyl-CoA equals 1.6 nmol/107 cells. D, hypoacetylation of bulk histones in plc1Δ cells is suppressed by mth1Δ mutation. Samples from the indicated strains were analyzed by Western blotting with antibodies against total histone H3, histone H3 acetylated at lysine 14 (acH3), and hyperacetylated histone H4 (acH4). Even loading of protein samples was confirmed with anti-phosphoglycerate kinase (Pgk1p) antibody. The experiment was performed three times, and representative results are shown. E, temperature sensitivity of plc1Δ is partially suppressed by mth1Δ mutation. 10-Fold serial dilutions of the indicated strains were spotted onto YPD plates, and grown for 2 days at 30 and 35 °C. Typical results from three independent experiments are shown. F, grr1Δ cells display global hypoacetylation of histones. Samples from the indicated strains were analyzed by Western blotting with antibodies against total histone H3, histone H3 acetylated at lysine 14 (acH3), and hyperacetylated histone H4 (acH4). Even loading of protein samples was confirmed with anti-phosphoglycerate kinase (Pgk1p) antibody. The experiment was performed three times, and representative results are shown.
