FIGURE 11.
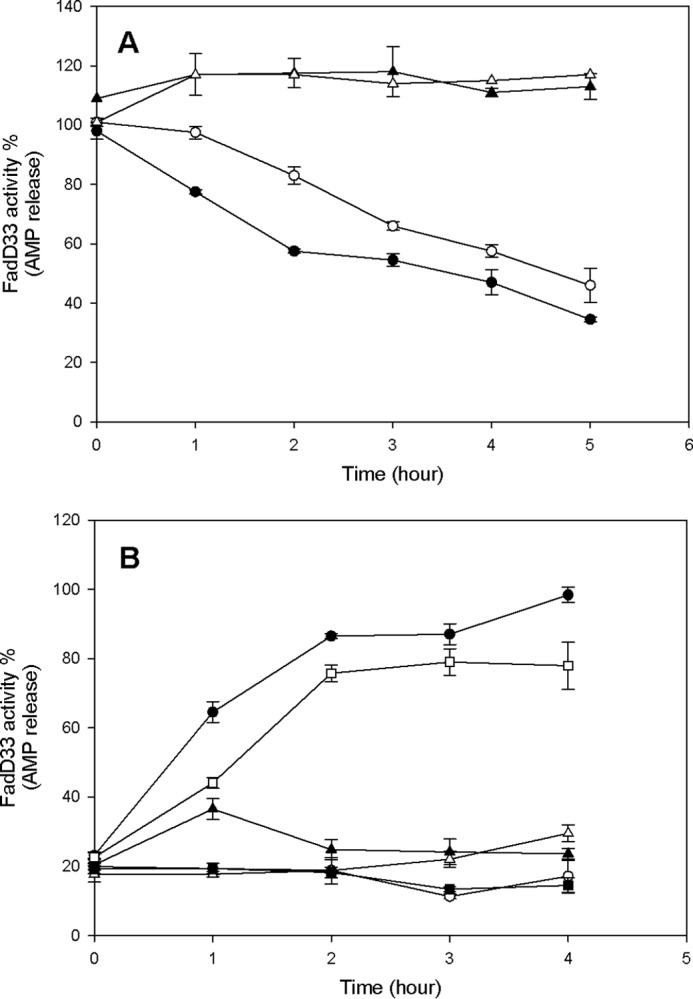
Effect of acetylation and deacetylation on FadD33 and FadD33-K260A activities. A, time-dependent inactivation of FadD33 and FadD33-K260A by acetylation. Enzymes activities were monitored at different time intervals with the following components: ● (FadD33), 1 mm cAMP, 5 μm Pat, and 100 μm acetyl-CoA; ○ (FadD33-K260A), 1 mm cAMP, 10 μm Pat, and 100 μm acetyl-CoA; ▴ (FadD33), 1 mm cAMP and 100 μm acetyl-CoA; ▵ (FadD33), 1 mm cAMP and 5 μm Pat. B, time-dependent reactivation of acetylated FadD33 and FadD33-K260A by deacetylation. After acetylation by Pat, FadD33, or mutant were then incubated with the following components: ● (Ac-FadD33), 2 mm NAD+, 2 μm DAc1; ○ (Ac-FadD33), 2 mm NAD+; ▴ (Ac-FadD33), 2 μm DAc1; ▵ (Ac-FadD33), 2 μm DAc2, 2 mm NAD+; □ (Ac-FadD33-K260A), 2 mm NAD+, 2 μm DAc1; and ■ (Ac-FadD33-K260A), 2 mm NAD+. Enzyme activities were monitored spectrophotometrically in 100 mm HEPES, pH 7.8, 10 mm MgCl2, and 250 mm NaCl as described under “Materials and Methods.”
