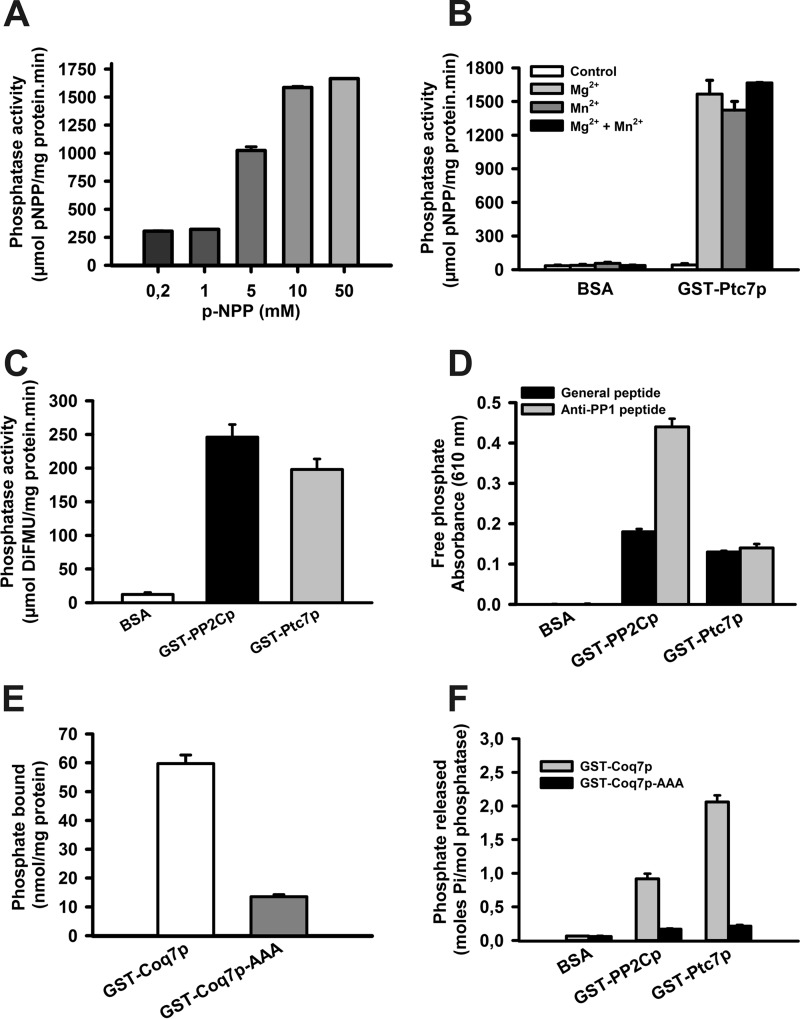
FIGURE 5.
Ptc7p dephosphorylates Coq7p in vitro. A, in vitro phosphatase assay using increasing concentrations of p-NPP. Activity is expressed as μmol of phosphate released/mg of protein·min. Error bars represent S.D. n = 3. B, Ptc7p activity without cofactors. An in vitro phosphatase assay using 40 mm p-NPP was performed to determine the requirement of magnesium and magnesium cofactors for Ptc7 phosphatase activity. 10 mm Mg2+ and 20 mm Mn2+ were added where indicated. BSA was used as a negative control. Activity is expressed as μmol of 6,8-difluoro-4-methylumbelliferyl (DiFMU) produced/mg of protein·min. Error bars represent S.D. n = 3. C, in vitro phosphatase assay using 1 mm DiFMUP as reagent with 0.5 μg of recombinant protein. As a positive control, the human recombinant phosphatase GST-PP2Cp was included. Error bars represent S.D. n = 3. D, phosphopeptide dephosphorylation assays. Data are expressed as increased absorbance at 610 nm using universal and anti-PP1 specific phosphopeptides. As a positive control, the human recombinant phosphatase GST-PP2Cp was included. Error bars represent S.D. n = 3. E, in vitro phosphorylation of versions of Coq7p. Recombinant proteins GST-Coq7p and GST-Coq7p-AAA (2 μg) were phosphorylated with [γ-32P]ATP and PKA. Total phosphorylation was directly determined in a scintillation counter and is expressed as nmol of bound phosphate/mg of protein. Error bars represent S.D. n = 3. F, in vitro Coq7p dephosphorylation. Recombinant proteins GST-Coq7p and GST-Coq7p-AAA were phosphorylated with [32P]ATP and PKA. Dephosphorylation by GST-PP2Cp, GST-Ptc7p, and BSA was determined after TCA precipitation. Activity is expressed as mol of phosphate released/mol of phosphatase. Error bars represent S.D. n = 3 in all experiments.