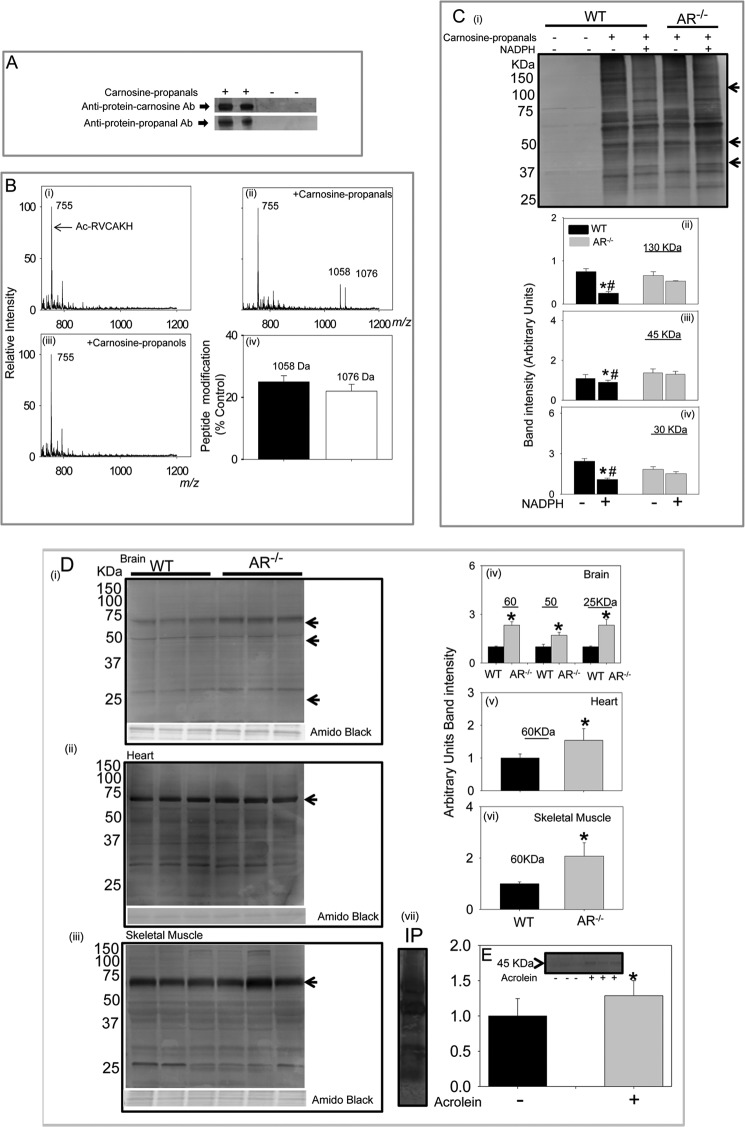
FIGURE 7.
Formation of protein-carnosine adducts. A, representative Western blots of albumin incubated with the carnosine-propanal. The blots were developed with either the anti-protein-carnosine or the anti-protein-propanal antibodies (Ab). B, ESI+/MS spectra of Ac-RVCAKH before (i) and after (ii) incubation with carnosine-propanal for 30 min or with carnosine-propanol for 60 min (iii) and percent modification of the model peptide by the carnosine-propanal conjugate (iv). C, Western blot of skeletal muscle lysates prepared from WT and AR-null mice incubated with 200–300 μm carnosine-propanal and 1 mm NADPH for 18 h developed using the anti-protein-carnosine antibody (i) and the relative intensity of bands of apparent molecular masses 130 (ii), 45 (iii), and 30 (iv) kDa normalized to total protein in the gel measured by Amido Black staining. Data are the mean ± S.E.; *, p < 0.01 versus WT + carnosine-propanal; #, p < 0.01 versus AR-null + carnosine propanal + NADPH (n = 3–4). D, accumulation of carnosinylated proteins in aged tissue from WT and AR-null mice. Total tissue lysates were prepared from the brain (i), heart (ii), and skeletal muscle (iii), and Western blots were developed using anti-protein-carnosine antibody. The relative intensity in specific immune-positive bands is shown in corresponding bar graphs (iv, v, and vi, respectively). vii, a representative Western blot developed using anti-propanal-protein antibody of skeletal muscle lysates immunoprecipitated (IP) with anti-protein-carnosine antibody. Data are expressed as the mean ± S.E. *, p < 0.01 versus WT tissues (n = 3–4). E, accumulation of carnosine-propanal-protein adducts in WT mouse hearts perfused with 10 μm acrolein (10 min). Blots were developed with anti-protein-carnosine antibody (inset). Data are expressed as mean ± S.E. *, p < 0.01 versus untreated hearts (n = 3–4).