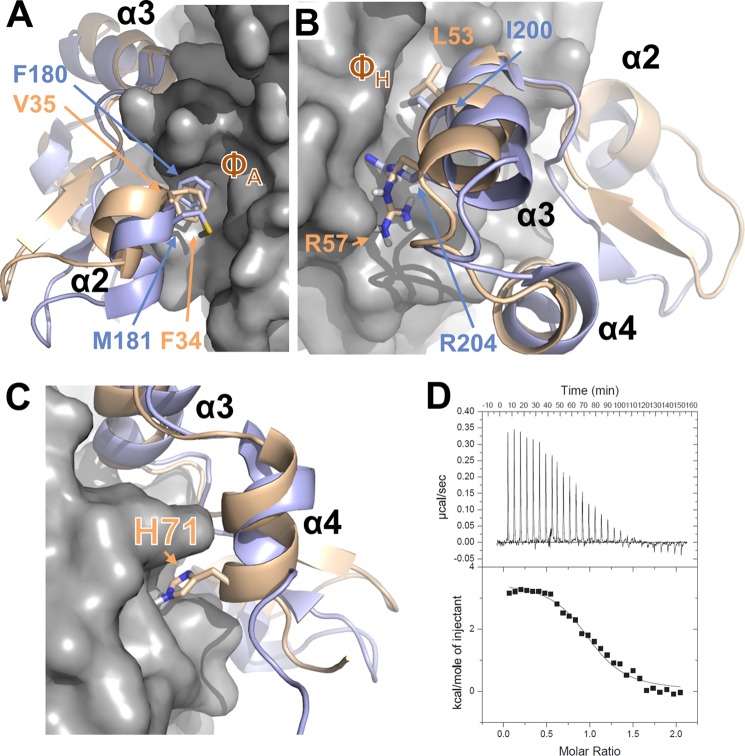
FIGURE 5.
Interaction of DUSP10 and DUSP16 with p38α is different. A, overlay of the p38α·DUSP16 MKBD complex (p38α is shown as gray surface; DUSP16 MKBD is shown as a wheat ribbon) with the p38α·DUSP10 MKBD complex (DUSP10 MKBD is shown as a light blue ribbon), illustrating the interaction of helix α2 with the p38α ΦA pocket. Key residues from DUSP10 and DUSP16 are shown as sticks and labeled. B, same as A but illustrating the interaction of helix α3 with the p38α ΦH pocket. Key residues from DUSP10 and DUSP16 are shown as sticks and labeled. C, same as A but illustrating the interaction of DUSP16 MKBD helix α4 with p38α. His-71 from DUSP16 is shown as sticks and labeled. D, raw isothermal titration calorimetry data (upper panels) and derived binding isotherm plotted versus the molar ratio of titrant fit using a one-site model (lower panels) for p38α with the DUSP16 MKBD H71A/S72A mutant.