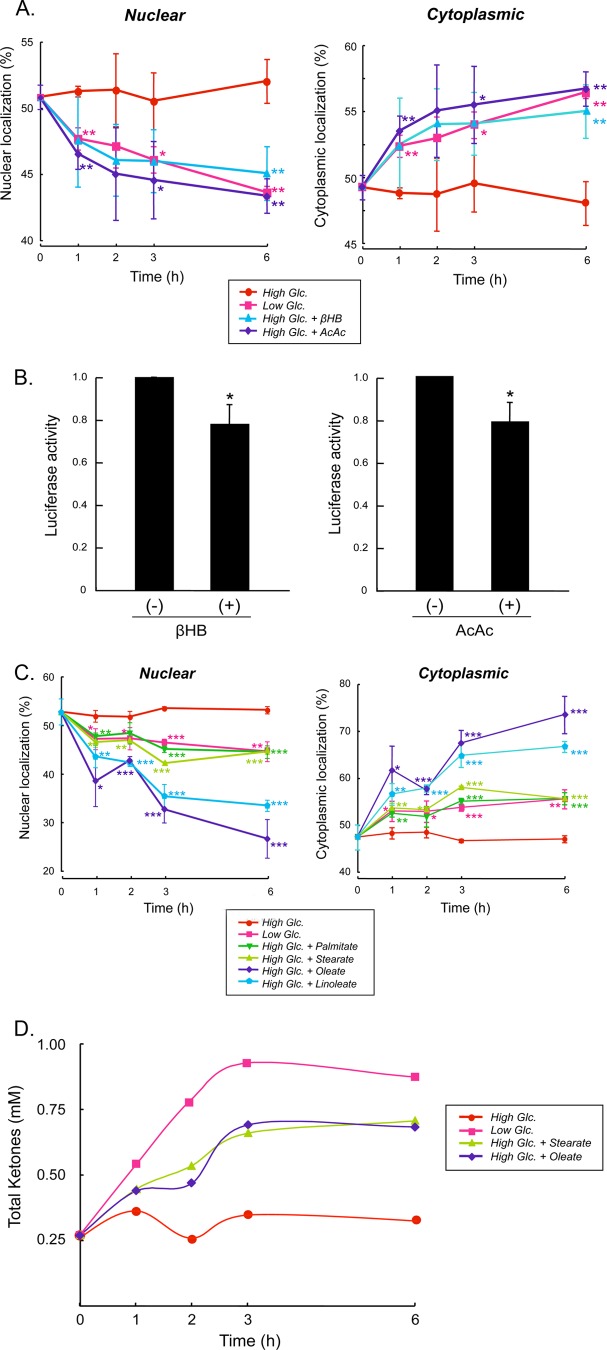
FIGURE 6.
βHB and AcAc inhibit nuclear localization of ChREBP. A, primary cultures of rat hepatocytes were transfected with GFP-ChREBP proteins in DMEM containing low (5.5 mm) glucose for 4 h and then the medium was changed to DMEM containing high (27.5 mm) glucose for 12 h and incubated in low (5.5 mm) or high (27.5 mm) glucose in the presence or absence of βHB (2 mm) or AcAc (2 mm). At the indicated time, the cells were fixed with 4% paraformaldehyde, and subcellular localization of GFP-ChREBP in the nucleus and cytoplasm was determined with using a confocal microscopy as described previously (5). The values presented are the mean ± S.D. of three sets of about 100 fluorescent cells. *, p < 0.05; **, p < 0.01 cf. high Glc. B, transcriptional activity in the hepatocytes incubated in βHB or AcAc were measured after 6 h using the luciferase reporter system as described (22) *, p < 0.05. C, fatty acids inhibit nuclear localization of ChREBP. Rat hepatocytes transfected with GFP-ChREBP were cultured in high glucose for 12 h, and the cells were incubated in the medium-containing stearate, palmitate, oleate, and linoleate, and cellular localization of GFP-ChREBP in nucleus and cytoplasm was determined at different time periods as above. The control cells were incubated in either low or high glucose. *, p < 0.05; **, p < 0.01; ***, p < 0.0001 cf. high Glc. D, βHB and AcAc produced by the cells incubated in the stearate and oleate were measured by removing the cells rapidly by centrifugation, and the packed cells were quickly frozen in liquid nitrogen. Acid extracts of the cells were prepared, and ketone bodies in the extracts were determined with GC-MS.