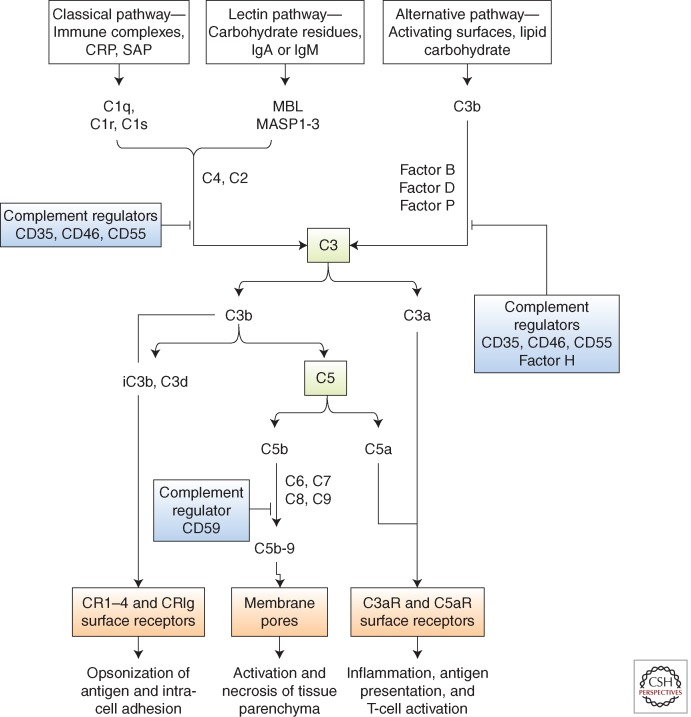
Figure 2.
The complement cascade. Complement is activated by three major pathways: classical, lectin, and alternative. The classical pathway is triggered by the binding of C1 to immune surveillance molecules that are attached to the target sequence (e.g., immunoglobulin), C-reactive protein (CRP), and serum amyloid protein (SAP). The lectin pathway is initiated by mannose binding protein (MBL), which binds to carbohydrate residues on the pathogenic surface or IgA and IgM molecules. The alternative pathway is triggered by direct binding of C3b to activating surface. All three pathways progress to form enzyme complexes (classical or alternative pathway) that convert C3 and then C5 into active forms. This generates three groups of complement effectors. C3b is highly reactive and attaches to the activating surface. C3b and metabolites iC3b and C3d are ligands for receptors that are found on leukocytes and mediate inflammation, antigen uptake, and B-cell stimulation. C5b triggers the formation of C5b-9, a multimeric complex that creates a pore in the target cell membrane and induces cell activation and cell death. The small peptide fragments C3a and C5a interact with receptors on leukocytes and parenchymal cells to promote inflammation and, in the presence of foreign antigen, enhance T-cell stimulation. Regulators of complement activation are soluble (e.g., factor H) or membrane-associated, for example, CD35 (complement receptor 1, CR1), CD46 (membrane cofactor protein, MCP), and CD55 (decay-accelerating factor, DAF). The regulators bind C3b (and C4b) and increase its decay or proteolysis from the C3 and C5 convertases of the classical and alternative pathways. Factor H is unique to the alternative pathway. Other regulators inhibit the formation of C5b-9 (e.g., through binding of C3 by CD59).