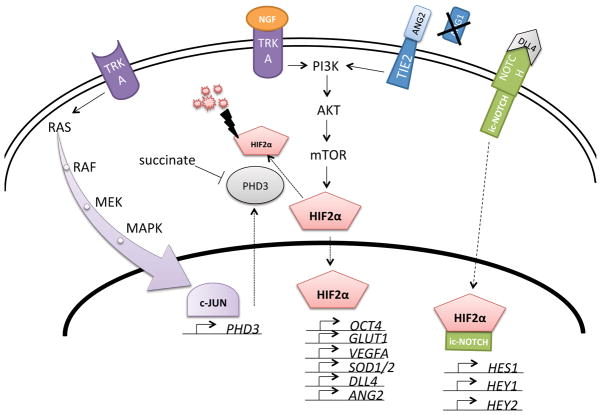
Figure 1. HIF2α signalling network in chromaffin cells.
Nerve growth factor (NGF) binding to its receptor tyrosine kinase TRKA induces PI3K/AKT signalling, which in turn activates the global translational regulator mTOR. One of its targets is hypoxia inducible factor 2α (HIF2α), a transcription factor able to induce a number of different genes, such as the NOTCH ligand delta-like ligand 4 (DLL4). HIF2α and intracellular NOTCH (ic-NOTCH) jointly activate genes, such as stem cell marker HES1. The HIF2α protein is labelled for degradation by prolyl hydroxylase 3 (PHD3) and processed by the proteasome complex, part of which is the Von Hippel- Lindau (VHL) protein. PHD3 is inhibited by high concentrations of succinate, which can be achieved by inactivation of the enzyme succinate dehydrogenase. When TRKA is not activated by NGF the RAS/MAPK pathway is activated instead leading to induction of the transcription factor c-JUN, which stimulates increased transcription of PHD3. An alternative way of PI3K activation is signalling via the TIE2 receptor, which is normally activated by its ligand angiopoietin-1 (ANG1). When angiopoietin-2 (ANG2) is present ANG1 effects can be inhibited, but when ANG1 is absent ANG2 is also able to induce TIE2 signalling.