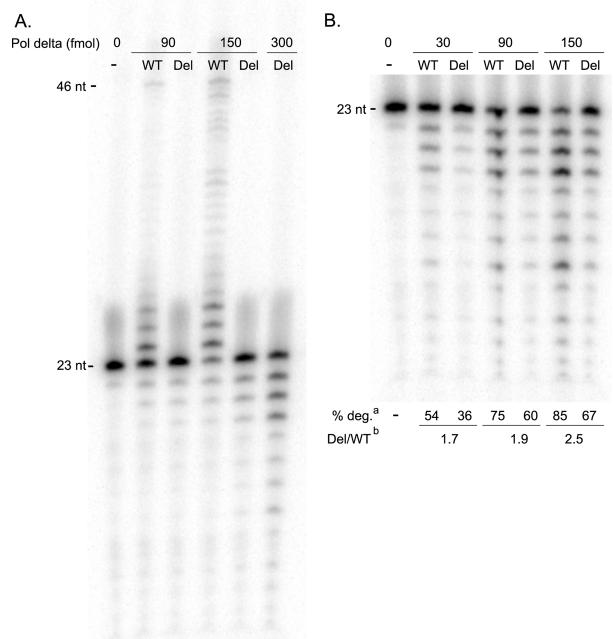
Figure 3. Pol delta S605del has no detectable polymerase activity, but robust exonuclease activity.
(a) Polymerase reactions using increasing concentrations of either wild type (WT) or S605del (Del) Pol delta reveal that the mutant is incapable of extending the primer band (23 nt) in a 2 minute reaction with 25 μM dNTPs. (b) Exonuclease reactions on the same 23/46 primed duplex DNA, in the absence of dNTPs, show that S605del exhibits a 2- to 3-fold decrease in exonuclease activity when compared to wild type. aValues for percent of primer band degraded (% deg.) reflect three independent experiments, and are derived from a comparison of the intensity of the primer band (P) and the exonuclease product bands (E), given by the following equation: % deg = 1-(P/(P+E)). bThe % degradation values of Pol delta-S605del at 90, 150, and 300 fmol were plotted against the line obtained from measurements with the wild type polymerase (y=0.0029+0.445; R2=0.96). The values derived indicate that ~1.7 to 2.5-fold more mutant enzyme is required to observe the same amount of primer degradation as the wild type enzyme under these experimental conditions.