Abstract
Umbilical cord care varies often reflecting community or health-worker beliefs. We undertook a review of current evidence on topical umbilical cord care. Study quality was assessed using the Grading of Recommendations, Assessment, Development and Evaluation system, and a meta-analysis was conducted for comparable trials. Available moderate-quality to high-quality evidence indicate that cord cleansing with 4% chlorhexidine may reduce the risk of neonatal mortality and sepsis (omphalitis) in low-resource settings.
Keywords: cord care, chlorhexidine, neonatal mortality, sepsis, neonates
Annually about 3.3 million neonatal deaths occur around the world;1 of these, more than 30% are caused by infections.2,3 Some of these infections start as umbilical cord infection. The umbilical cord area supports growth of some innocuous or beneficial microorganisms (commensals) whereas others are harmful (eg, Clostridium tetani). Sources of these bacteria include the mother’s birth canal, the environment in which the neonate is delivered and hands of the person assisting with the delivery. Cord infection may be localized to the umbilical cord (omphalitis) or, after entry into the blood stream, become systemic (eg, neonatal sepsis).
Data on the incidence of omphalitis in low-income countries is generally scarce, the available data estimate the risk to range between 2 and 77 per 1000 live births in hospital settings, with fatality rates of between 1% and 15% depending on the definition of omphalitis used.4 Community-based data show even higher infection rates: for example, 105 per 1000 live births in Nepal,5 217 per 1000 live births in Pakistan and about 197 per 1000 live births in India.4 Remarkably, no data are currently available from most countries in Africa where most deliveries still occur at home and where neonatal mortality remains high.2 Two trials on the effect of 4% chlorhexidine (CHX) on neonatal mortality and umbilical cord infections are, however, ongoing in Zanzibar6 and Zambia.7
As cord infections should be preventable in most cases,8 it is important to identify best cord care practices to reduce neonatal mortality and morbidity and offer an alternative to widespread potentially harmful traditional practices. Examples of such practices include use of traditional herbs mixed with cooking oil or water that has been used to wash an adult woman’s genitals (numbati) or application of ash, breast milk, fluid from pumpkin flowers, powder ground from local trees, cow dung, ghee and saliva that may be applied to the cord area and which may be harmful.9,10
Internationally, the World Health Organization has advocated since 1998 for the use of dry umbilical cord care (keeping the cord clean without application of anything and leaving it exposed to air or loosely covered by a clean cloth, in case it becomes soiled it is only cleaned with water). World Health Organization recommends topical antiseptics (eg, CHX) in situations where hygienic conditions are poor and/or infection rates are high.11 However, a Cochrane review12 (n = 8959 newborns, 21 studies) published in July 2004 found no benefit on neonatal mortality or rates of disseminated or localized infection of applying antiseptics or antibiotics to the cord stump compared with dry cord care. The findings of this Cochrane review may, however, not be generalizable to the African setting, as most of the included studies (19/21) were from high-income countries and all but 1 were conducted in hospital settings.
In Kenya, clinical guidelines recommend dry cord care,13,14 however, anecdotal evidence and experience suggest that health care providers vary in their practice, for example, using alcohol, methylated spirit or povidone iodine to clean the cord. To help Kenyan policy makers develop appropriate national guidance, we conducted a review to define safe and effective topical umbilical cord care for prevention of mortality and cord infections in newborn infants.
METHODS
Search Strategy
Potential studies for inclusion in the review were identified through direct searches on the Cochrane Library and PubMed. The search terms used were “umbilical cord” OR “umbilical” OR “umbilicus” AND “neonate” OR “newborn” OR “infant” AND “anti-bacterial agent” OR “anti-infective agent” OR “anti-sepsis agent” OR “antimicrobial agent” OR “antiseptic”. PubMed clinical query filters for systematic reviews and randomized controlled trials (RCTs) were applied. We searched for studies published between January 2003 and February 2012 (spanning the period not included in the Cochrane review12 published in July 2004). Bibliographic references of retrieved reviews and studies were searched for additional articles. The searches were limited to studies published in English due to time constraints.
Study Selection Criteria
RCTs and cluster-RCTs (cRCTs) that compared different topical agents versus dry cord care, different cord cleansing antiseptics and single versus multiple use of cord cleansing antiseptics in newborn babies (both term and preterm) were eligible for inclusion. Community and hospital-based studies from low-income, middle-income and high-income countries were eligible for inclusion. Observational studies were excluded. The prespecified critical outcomes were neonatal mortality (deaths within the first 28 days of life), neonatal sepsis originating from cord infection and omphalitis (redness or swelling of the cord with or without pus within the first 28 days of life). Cord separation time was considered an important outcome as delay in separation has not been shown to be associated with adverse consequences although it may be of concern to parents and health workers.
All titles and abstracts of identified articles were screened by 3 reviewers independently and subjected to the prespecified study selection criteria; any disagreements were resolved by discussion.
Data Extraction
Data on study characteristics (study design, settings), participants, nature of interventions, proportion of participants with events of interest (eg, death or infection of the cord stump) and outcome measures were extracted and entered into a standardized form. The abstraction was done by 1 reviewer; a second reviewer counter checked the extracted data. Disagreements were resolved by consensus.
Data Synthesis
For the studies published after the Cochrane review,12 we summarized results narratively for a subset of hospital-based studies where interventions and outcomes varied significantly. However, for 3 cRCTs, a random-effects meta-analysis (studies had some heterogeneity) was used to calculate pooled risk ratios (RRs) and 95% confidence intervals (CIs) for mortality outcomes. χ2 test of heterogeneity and I2 statistic were used to assess and quantify heterogeneity.
Quality Appraisal
The quality of the available evidence about the prespecified outcomes to support a given intervention was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.15 The GRADE approach classifies evidence quality into 4 categories: high, moderate, low or very low. The unique features of GRADE include: (1) explicit, comprehensive criteria for downgrading and upgrading quality of evidence ratings; (2) explicit evaluation of the importance of outcomes; and (3) clear separation of quality of evidence from the strength of recommendations. Results of the quality assessments are summarized and presented using GRADE summary-of-findings tables. Two reviewers independently assessed study quality; disagreements were resolved by discussion. Studies were not excluded based on their quality rating but outcomes of quality assessments were taken into account in the synthesis of results.
RESULTS
Forty-four potentially relevant articles were identified from the literature searches. Eight studies16-23 and 2 systematic reviews14,24 were deemed eligible for inclusion (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/B375). Study settings varied; 3 studies were conducted in low-income countries, 2 in middle-income countries and 3 in high-income countries. The interventions used included 4% CHX, olive oil, triple dye, breast milk, alcohol and silver sulfadiazine (antibiotic) whereas the comparisons included dry cord care and salicylic sugar (Table, Supplemental Digital Content 2, http://links.lww.com/INF/B376). Quality of evidence for the reported outcomes varied from (low to high; Tables 1-3).
TABLE 1.
Summary-of-findings Table on Mortality and Omphalitis
| Illustrative Comparative Risks |
||||||
|---|---|---|---|---|---|---|
| Outcomes | Assumed Risk |
Corresponding Risk |
Relative Effect (95% CI) |
No. of Participants (Studies) |
Quality of the Evidence (GRADE) |
Comments |
| Dry Care | Antimicrobials | |||||
| Neonatal mortality number of deaths within 28 d |
26 per 1000 | 23 per 1000 (21–26) | RR 0.88 (0.79–0.99) | 44,818 (3 studies) | ⊕⊕⊕⊕ high* | |
| Follow-up: mean 28 d | ||||||
| Omphalitis number of omphalitis cases |
108 per 1000 | 84 per 1000 (81–88) | RR 0.78 (0.75–0.81) | 44,600 (3 studies) | ⊕⊕⊕⊕⊖ moderate* | |
| Follow-up: mean 28 d | ||||||
Antimicrobials compared with dry care for umbilical cord care.
Patient or population: patients with umbilical cord care
Settings: community
Intervention: antimicrobials
Comparison: dry care
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Study setting: Asia.
TABLE 2.
Summary-of-findings Table on Sepsis and Omphalitis
| Illustrative comparative risks |
||||||
|---|---|---|---|---|---|---|
| Outcomes | Assumed Risk |
Corresponding Risk |
Results | No. of Participants (studies) |
Quality of the Evidence (GRADE)v |
Comments |
| Dry Care/Other Antimicrobials |
Antimicrobial Agent | |||||
| Omphalitis (infection) number of cases |
Not estimable | Not estimable | Use of antimicrobials has no effect on cord infection in the hospital setting |
822 (4 studies†) | ⊕⊕⊖⊖ low*†‡§ | |
| Follow-up: mean 6 weeks |
||||||
Antimicrobial agent compared with dry care/other antimicrobials for umbilical cord care.
Patient or population: newborn babies
Settings: hospital
Intervention: antimicrobial agent
Comparison: dry care/other antimicrobials
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
All sample sizes less than 350; all studies were hospital based.
One study(Pezzati et al, 2003)20 compared a powder vs. a solution hence blinding was not possible.
All the studies used different interventions ranging, including olive oil, salicylic powder, alcohol, breast milk.
Study settings: hospital setting in middle-income and high-income countries.
TABLE 3.
Summary-of-findings on Umbilical Cord Separation Time (UCST)
| Illustrative comparative risks |
||||||
|---|---|---|---|---|---|---|
| Outcomes | Assumed risk |
Corresponding risk |
Results | No. of Participants (studies) |
Quality of the evidence (GRADE) |
Comments |
| Dry care | Antimicrobial | |||||
| Umbilical cord sepa- ration time (time in days) |
Not estimable | The mean umbili- cal cord separa- tion time in the intervention group ranged from 4 to 17 higher |
UCSTs ranged from 4 to 17 d |
25,256 (5 studies) | ⊕⊕⊖⊖ low*† | |
Antimicrobial use in umbilical cord care
Patient or population: newborn babies
Settings: both community and hospital
Intervention: antimicrobial
Comparison: dry care
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Study settings: cluster-RCTs were in low-income countries community setting whereas the 3 small RCTs were in middle-income and high-income countries.
Two studies were huge cluster-RCTs (N = 24,628) whereas the other 3 were small RCTs (N = 628).
Systematic Reviews
One Cochrane review12 published in July 2004 summarized the findings of 21 hospital studies (13 RCTs and 8 quasi-RCTs; N = 8959). Nineteen of the studies were conducted in high-income countries, and only 2 were from low-resource countries. Overall, the review concluded that topical cord care with the various agents (antiseptics, antibiotics or even traditional substances) was not superior to air drying of the cord for the outcomes of mortality or systemic infections originating from the cord. None of the reviewed studies included any fatal events or episodes of severe sepsis. However, the reviewers acknowledged that the results of the primary studies were not generalizable to the global newborn population as there were few (2 of 21; n = 699 newborns) studies from low-resource settings. The GRADE approach was, however, not used to assess the quality of evidence in this review.
The non-Cochrane review24 published in November 2003 examined the use of antimicrobial agents to reduce bacterial colonization and local infection (omphalitis) in newborn infants. Eighteen studies (10 RCTs and 8 observational designs) enrolling a total of 39,635 newborns were included. None of the studies reported mortality among the participants. Ten studies had infection as an outcome (3 RCTs, n = 3540 and 7 observational studies, n = 32,057): 2 of the RCTs (n = 2774) and 3 observational studies (n = 7704) found 4% CHX to be associated with decreased rates of infections. The remaining studies compared alternative topical antimicrobials or topical antimicrobials with dry cord care and reported no significant difference in infection rates (both local and systemic). Overall, the authors concluded that in areas with high infection rates cord antisepsis (particularly 4% CHX) has the potential to reduce the risk of neonatal sepsis and death (although none of the included studies reported any deaths). They also acknowledged that antimicrobials reduced colonization but there has been no evidence to show that increased colonization of the umbilical stump leads to infections.
Randomized Controlled Trials
The results of 5 hospital-based and 3 community-based trials published after the included Cochrane review14 are summarized below.
Effects of 4% Chlorhexidine on Neonatal Mortality
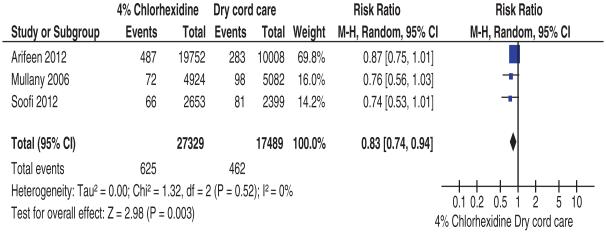
Four studies assessed this outcome. Three of these studies were community-based cRCTs,16,18,22 and 1 was a hospital-based RCT.21 The 3 high-quality cRCTs (n = 44,818) used 4% CHX as the intervention compared with dry care (Table 1). Meta-analysis of these 3 studies showed that 4% CHX, compared with dry care, was associated with a 17% reduction in the risk of neonatal mortality (pooled RR 0.83, 95% CI: 0.74–0.94, I2 = 0%; Fig. 1). Arifeen et al18 (n = 29,760) also compared the effect of single 4% CHX application (as soon as possible after birth) and multiple CHX cleansing (daily cleansing for 7 days after birth) to dry cord care. Neonatal mortality was significantly reduced in the single CHX cleansing group compared with the dry cord care group (RR 0.80, 95% CI: 0.65–0.98). No differences in neonatal mortality were found between multiple CHX cleansing and dry cord care groups (RR 0.94, 95% CI: 0.78–1.14). The hospital-based study21 (n = 244) compared the effects of 4% CHX and salicylic sugar powder on mortality; no mortality was reported in either of the comparison groups.
FIGURE 1.
Comparison: 4% chlorhexidine versus dry cord care, outcome: all-cause neonatal mortality.
Effects on Neonatal Sepsis and Omphalitis
Omphalitis was defined as severe redness and pus in 2 studies14,18 and any redness and pus in 1 study.22 The hospital-based studies17,19-21,23 had no clear definition of omphalitis.
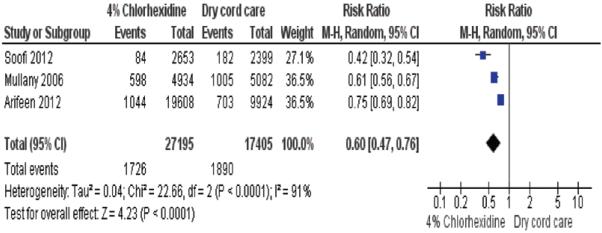
This outcome was reported by 3 community and 5 hospital studies (N = 55,334). Moderate quality evidence (Table 1) from the 3 community-based studies suggests a reduction in omphalitis and sepsis with the use of 4% CHX. Soofi et al22 (n = 9741) reported a reduction of omphalitis in the CHX cleansing group (RR 0.44, 95% CI: 0.29–0.67) whereas Mullany et al16 (n = 14,887) reported a reduction by 32–75% depending on the definition of the omphalitis (severe redness and pus: RR 0.25, 95% CI: 0.12–0.53; pus and moderate or severe redness or severe redness alone: RR 0.46, 95% CI: 0.36–0.56; redness extending to base of umbilicus: RR 0.68, 95% CI: 0.58–0.80). This reduction of severe omphalitis was even bigger if the intervention was within 24 hours of birth (incidence rate ratio 0.13, 95% CI: 0.07–0.31). Arifeen et al18 (n = 29,760) reported a reduction in severe infection (redness with pus) in the multiple CHX cleansing group (RR 0.35, 95% CI: 0.15–0.81) and a lesser reduction in the single CHX cleansing group (RR 0.77, 95% CI: 0.40–1.48).
Low-quality evidence from small hospital-based studies17,19-21,23 (N = 946) suggests that use of antimicrobials has no effect on cord infection (Table 2). Pezzati et al21 (Italy, n = 244) compared 4% CHX with salicylic sugar powder in preterm babies but reported only 1 case of sepsis in each arm, whereas Ahmadpour et al17 (Iran, n = 312), Erenel et al19 (Turkey, n = 150), Hsu et al20 (Taiwan, n = 150) and Suliman et al23 (United States, n = 90) reported no or very few cases of omphalitis or sepsis when newborns were randomized to cord care approaches including breast milk, 96% alcohol, silver sulfadiazine, triple dye, olive oil or dry cord care.
Effects on Umbilical Cord Separation Times in Days
Six trials5,17,19,20,22,23 (N = 25,400) reported on this outcome. The quality of evidence for this outcome was rated as low (Table 3). Overall, mixed results were reported with the use of some antimicrobials (eg, silver sulfadiazine and CHX) prolonging the time to cord separation whereas others (eg, breast milk and alcohol) shortened it. In addition, type of formulation was reported to play a major role in that powdered formulations tended to reduce separation time whereas liquid formulations tended to prolong it when compared with dry cord care. Overall, the mean umbilical cord separation time ranged from 4 to 16 days depending on the intervention and study setting (Table, Supplemental Digital Content 3, http://links.lww.com/INF/B377.).
DISCUSSION
High-quality evidence from 3 community cRCTs, in rural areas of low-income countries, enrolling about 44,000 newborns suggests that the use of 4% CHX reduces neonatal mortality in community settings, more so if this intervention is used in regions where infection rates are high. Although data are from Asia, such settings are likely similar to several sub-Saharan countries including Kenya, where home delivery is common and infection rates probably high. Use of 4% CHX was found to be more effective if offered within the first 24 hours, and it seems that even a single application is effective. This may be explained by the activity of CHX, an antiseptic that has a long duration of action and is broad spectrum.11 In contrast, hospital-based trials that were generally much smaller with low event rates provide low-quality evidence and did not report any effect of antimicrobials on mortality.
The heterogeneous nature of the data on sepsis/omphalitis outcomes made it inappropriate to combine the results in a meta-analysis (I2 = 91%, Fig. 2). The causes of this heterogeneity were explored and included differences in: the frequency of CHX applications, definitions of infections (omphalitis) and study designs. However, there was moderate quality evidence suggesting that 4% CHX greatly reduces morbidity especially when started within 24 hours of birth in community settings and low-quality evidence (Table 2) suggesting that applying an antimicrobial is no better than leaving the cord to dry when in the high-income or middle-income hospital setting. The apparent consistent evidence of effect, at least in community settings, of 4% CHX contrasts with the mixed effects noted when it has been used by application to the mother’s birth canal or more widely to the skin surface of neonates.25,26
FIGURE 2.
Comparison: 4% chlorhexidine versus dry cord care, outcome: neonatal sepsis and omphalitis.
Low-quality evidence (Table 3) suggests that application of a powder formulation reduces the time to cord separation. This is consistent with other studies not included in this review27-29 that report that powder formulations reduce cord separation time, attributed to a drying effect, although they may lead to cord bleeding. These findings may be of relevance in selecting CHX formulation if health workers and families prefer more rapid cord separation.
In addition to considering the effectiveness of CHX, its safety is important when wide-scale use is proposed. Fortunately, CHX is felt to be generally safe to use as despite extensive use in medical settings since the 1950s, only isolated adverse events such as contact sensitivity, dermatitis, urticaria and photosensitivity have been reported. There have been specific, extensive studies of the safety of CHX in newborns. Although reports have documented percutaneous absorption of CHX when used as a body wash or for cord care, no adverse clinical consequences have been reported. This absorption is, however, likely to occur when CHX is used on premature and underweight newborns.30 None of the 3 cRCTs recorded any severe adverse event further supporting CHX’s safety profile.
There are some limitations to our study. First, we searched only a limited number of databases, with a focus on English language publications, but our review included data on a large number of newborns (N = 54,000). Second, none of the studies so far reported are from African settings making downgrading of the quality of evidence on the basis of indirectness a consideration from a Kenyan perspective. Sparser, lower quality data suffering more obviously from limitations of indirectness were challenges when considering CHX use for facility-based births. For a country such as Kenya, where omphalitis and severe infection in facility-born babies may still be common, arguably data from both community and facility settings should be used to inform discussions on recommendations. Finally, application of other potentially harmful substances in the dry cord care group may have confounded group comparisons.
Based on the high-quality evidence from the included studies, it seems reasonable to include 4% CHX for topical cord care as a package of care for clean deliveries for newborn infants both in the community and hospital settings in Kenya and similar countries in anticipation of positive results from studies already under way in Zanzibar6 and Zambia.7 Such studies are expected to provide additional information on the frequency of application. Although CHX is itself relatively cheap (the cost is about $9 per 5 liters in Kenya),31 thought will need to be given to the logistics of delivering such an intervention routinely in countries where at least half of all births occur at home in the absence of a skilled attendant. Logistic considerations will benefit from research on optimal formulations (such as gels or powders rather than liquids), delivery channels and professional and public education to stimulate demand and promote uptake of CHX.
Based on the available moderate-quality to high-quality evidence, we conclude that the use of 4% CHX can be beneficial in reducing neonatal mortality and morbidity among infants born at home in countries such as Kenya. For infants born in hospitals, only low-quality evidence largely from high-income settings currently exists and shows no benefit of using any topical substance including CHX over dry cord care. Given that babies born in facilities in low-income African settings have moderately high risks of severe infection and to promote development of a consistent, systemwide policy, it seems reasonable to consider using 4% CHX for all births in Kenya. More research is needed on the optimal mode of CHX delivery to support its use at scale.
Supplementary Material
ACKNOWLEDGMENTS
This work is published with the permission of the Director of KEMRI.
JK, MM and JA were supported by funds from the SIRCLE Collaboration. ME was supported by a Wellcome Trust Senior Fellowship (#076827). NO was supported by funds from a Wellcome Trust Strategic Award (#084538). The funding source had no role in the conduct of the review and writing of the report. The authors have no other funding or conflicts of interest to disclose.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Oestergaard MZ, Inoue M, Yoshida S, et al. United Nations Inter-Agency Group for Child Mortality Estimation and the Child Health Epidemiology Reference Group Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8:e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Mullany LC, Darmstadt GL, Katz J, et al. Risk of mortality subsequent to umbilical cord infection among newborns of southern Nepal: cord infection and mortality. Pediatr Infect Dis J. 2009;28:17–20. doi: 10.1097/INF.0b013e318181fb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir F, Tikmani SS, Shakoor S, et al. Incidence and etiology of omphalitis in Pakistan: a community-based cohort study. J Infect Dev Ctries. 2011;5:828–833. doi: 10.3855/jidc.1229. [DOI] [PubMed] [Google Scholar]
- 5.Mullany LC, Darmstadt GL, Khatry SK, et al. Impact of umbilical cord cleansing with 4.0% chlorhexidine on time to cord separation among newborns in southern Nepal: a cluster-randomized, community-based trial. Pediatrics. 2006;118:1864–1871. doi: 10.1542/peds.2006-1091. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed March 26, 2012];Chlorhexidine cordcare for reduction in neonatal mortality and omphalitis (CHX-Pemba) ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT01528852.
- 7. [Accessed March 26, 2012];Impact of chlorhexidine cord cleansing for prevention of neonatal mortality in Zambia. ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/related/NCT01241318.
- 8.Capurro H. Topical umbilical cord care at birth: RHL commentary. The WHO Reproductive Health Library; 2004. [Google Scholar]
- 9.Mrisho M, Schellenberg JA, Mushi AK, et al. Understanding home-based neonatal care practice in rural southern Tanzania. Trans R Soc Trop Med Hyg. 2008;102:669–678. doi: 10.1016/j.trstmh.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Mullany LC, Darmstadt GL, Katz J, et al. Risk factors for umbilical cord infection among newborns of southern Nepal. Am J Epidemiol. 2007;165:203–211. doi: 10.1093/aje/kwj356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Care of the umbilical cord. Maternal and new born health/safe motherhood. World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
- 12.Zupan J, Garner P, Omari AA. Topical umbilical cord care at birth. Cochrane Database Syst Rev. 2004:CD001057. doi: 10.1002/14651858.CD001057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Public Health and Sanitation, Kenya . National Guidelines on Essential Newborn Care. [Google Scholar]
- 14.Ministry of Health Kenya . Clinical Guidelines 2009; I and II. [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367:910–918. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadpour-Kacho M, Zahedpasha Y, Hajian K, et al. The effect of topical application of human milk, ethyl alcohol 96%, and silver sulfadiazine on umbilical cord separation time in newborn infants. Arch Iran Med. 2006;9:33–38. [PubMed] [Google Scholar]
- 18.Arifeen SE, Mullany LC, Shah R, et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet. 2012;379:1022–1028. doi: 10.1016/S0140-6736(11)61848-5. [DOI] [PubMed] [Google Scholar]
- 19.Erenel AS, Vural G, Efe SY, et al. Comparison of olive oil and dry-clean keeping methods in umbilical cord care as microbiological. Matern Child Health J. 2010;14:999–1004. doi: 10.1007/s10995-009-0536-4. [DOI] [PubMed] [Google Scholar]
- 20.Hsu WC, Yeh LC, Chuang MY, et al. Umbilical separation time delayed by alcohol application. Ann Trop Paediatr. 2010;30:219–223. doi: 10.1179/146532810X12786388978643. [DOI] [PubMed] [Google Scholar]
- 21.Pezzati M, Rossi S, Tronchin M, et al. Umbilical cord care in premature infants: the effect of two different cord-care regimens (salicylic sugar powder vs chlorhexidine) on cord separation time and other outcomes. Pediatrics. 2003;112:e275. doi: 10.1542/peds.112.4.e275. [DOI] [PubMed] [Google Scholar]
- 22.Soofi S, Cousens S, Imdad A, et al. Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. Lancet. 2012;379:1029–1036. doi: 10.1016/S0140-6736(11)61877-1. [DOI] [PubMed] [Google Scholar]
- 23.Suliman AK, Watts H, Beiler J, et al. Triple dye plus rubbing alcohol versus triple dye alone for umbilical cord care. Clin Pediatr (Phila) 2010;49:45–48. doi: 10.1177/0009922808329455. [DOI] [PubMed] [Google Scholar]
- 24.Mullany LC, Darmstadt GL, Tielsch JM. Role of antimicrobial applications to the umbilical cord in neonates to prevent bacterial colonization and infection: a review of the evidence. Pediatr Infect Dis J. 2003;22:996–1002. doi: 10.1097/01.inf.0000095429.97172.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutland CL. Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet. 2009;374:1909–1916. doi: 10.1016/S0140-6736(09)61339-8. [DOI] [PubMed] [Google Scholar]
- 26.Bakr AF, Karkour T. Effect of predelivery vaginal antisepsis on maternal and neonatal morbidity and mortality in Egypt. J Womens Health (Larchmt) 2005;14:496–501. doi: 10.1089/jwh.2005.14.496. [DOI] [PubMed] [Google Scholar]
- 27.Pezzati M, Biagioli EC, Martelli E, et al. Umbilical cord care: the effect of eight different cord-care regimens on cord separation time and other outcomes. Biol Neonate. 2002;81:38–44. doi: 10.1159/000047182. [DOI] [PubMed] [Google Scholar]
- 28.Arad I, Eyal F, Fainmesser P. Umbilical care and cord separation. Arch Dis Child. 1981;56:887–888. doi: 10.1136/adc.56.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapellen TM, Gebauer CM, Brosteanu O, et al. Higher rate of cord-related adverse events in neonates with dry umbilical cord care compared to chlorhexidine powder. Results of a randomized controlled study to compare efficacy and safety of chlorhexidine powder versus dry care in umbilical cord care of the newborn. Neonatology. 2009;96:13–18. doi: 10.1159/000200165. [DOI] [PubMed] [Google Scholar]
- 30.Mullany LC, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25:665–675. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenya Medical Supplies Agency . Drugs prices. KEMSA—Commodity Catalogue; [Accessed March 26, 2012]. Available at http://www.kemsa.co.ke/datagrids/kemsa_commodity_catalogue.php. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.