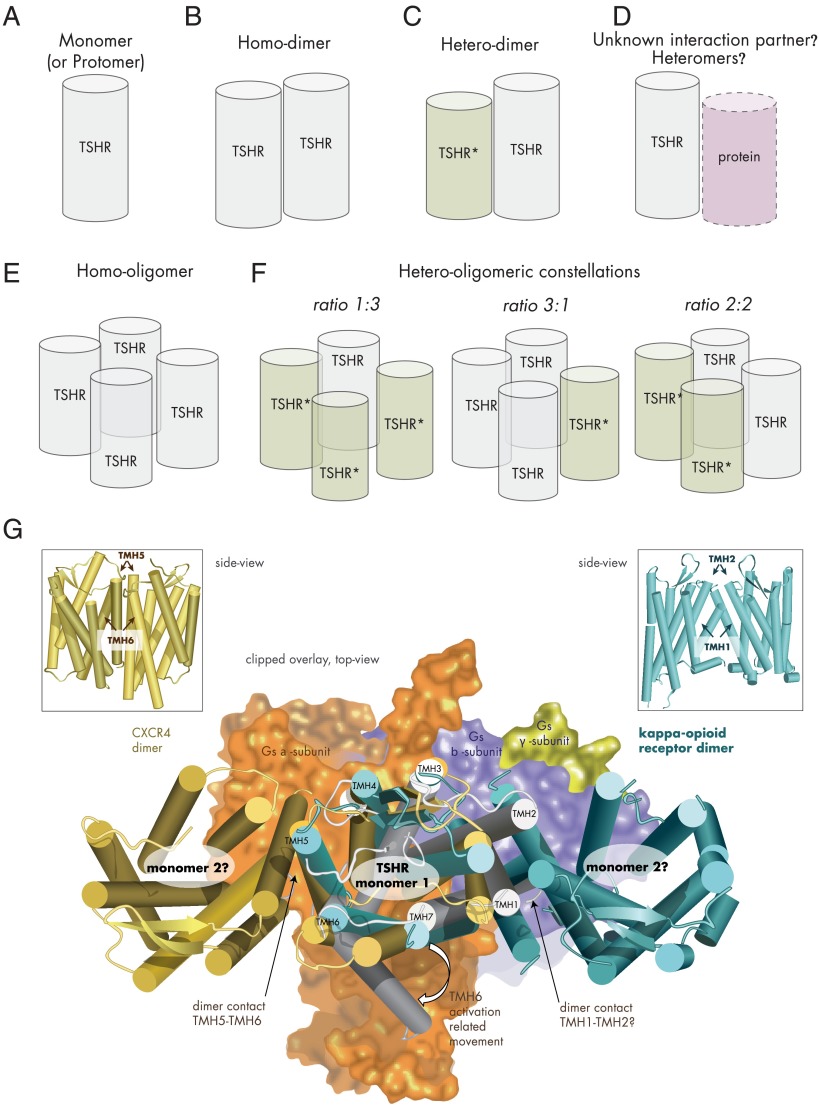
Figure 5.
Potential TSHR constellations. A, TSHR as monomer (schematic cylinder indicates the entire receptor). B, Two monomers interacting as TSHR homodimer. C, Dimerization of wild-type and mutant TSHR (TSHR*), which can result in a dominant-negative effect for heterozygous inactivating mutations (57). D, Potentially, the TSHR might be able also to interact with as yet unknown membrane-spanning proteins. E, The TSHR may also form homo-oligomers with different interfaces between the monomers. F, Assuming tetrameric (or higher-order) TSHR oligomers (373, 418), complexes with different monomer ratios could be formed from mutated variants and wild-type receptor: 1) wild type receptors only, 2) mutant receptors only, 3) 1 wild-type and 3 mutant receptors, 4) 2 wild-type and 2 mutant receptors or 3 wild-type and 1 mutant receptor. G, The crystallized complex between the ADRB2 and the heterotrimeric Gs protein pointed to significant structural differences between the inactive and active GPCR conformations (101). Especially, TMH6 is moved toward the membrane during G protein activation (active TSHR model [Figure 3], gray background, arrow at TMH6). Combining a monomeric active-state TSHR model coupled with Gs (subunits as surface), based on the crystallized ADRB2/Gs complex (101), with dimeric GPCR structures (CXCR4, dark-yellow) (388), and the κ-opioid receptor (light blue) (389) by structural overlay makes it obvious that in putative TSHR monomer-monomer complexes according to these crystal structure arrangements, coupling of 2 large activated bulky G protein molecules simultaneously at each receptor monomer would be sterically hindered, which would lead to asymmetric G protein binding.