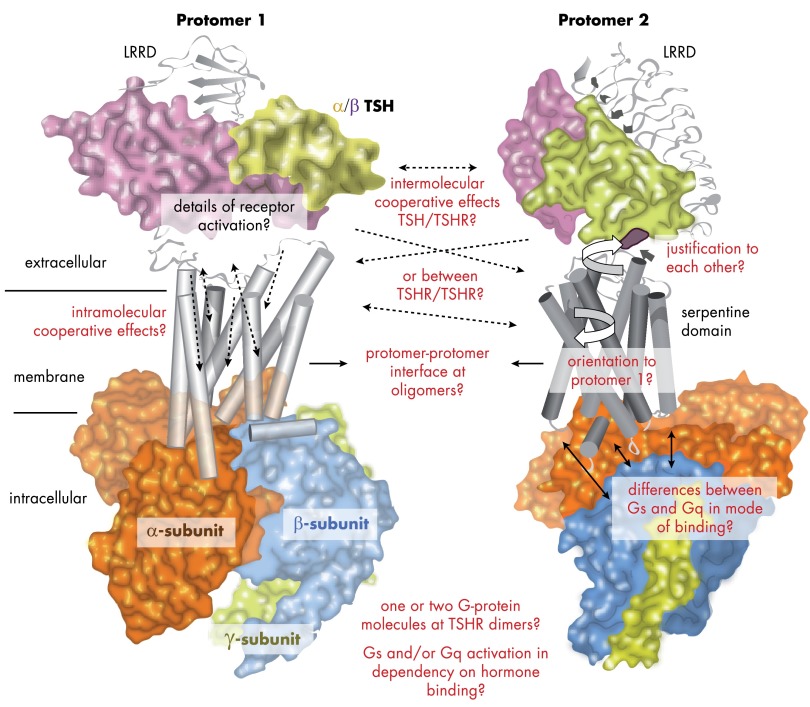
Figure 6.
Questions that shall be answered by additional studies on TSHR structure and function visualized at models of signaling complexes. As pointed out throughout this review, specific questions regarding the mechanisms of TSHR signaling are still awaiting answers. These topics are related to complex situations, i.e., binding of the G protein subtypes, the arrangement and justification of receptor components relative to each other, and the aspects of a dimeric or oligomeric state of the TSHR. So far, it is unknown how receptor components like the hinge region and the SD interrelate to each other or how they influence their function mutually. The same is true for the potential interaction interface between 2 receptor molecules. Strikingly, these missing data hamper a detailed determination of components that transfer known synergistic effects. The signal transduction process at the TSHR leads to intracellular activation of signaling pathways. Therefore, molecular events will be better understood if we will be able to answer the following questions. Why does the TSHR express a permanent basal signaling activity? Do 1 or 2 G protein molecules bind at a receptor dimer? Is there a relation between monomeric and dimeric constellation and G protein selectivity? Answers to these questions will allow a better explanation of pathogenic constellations on the molecular level of TSHR activation.