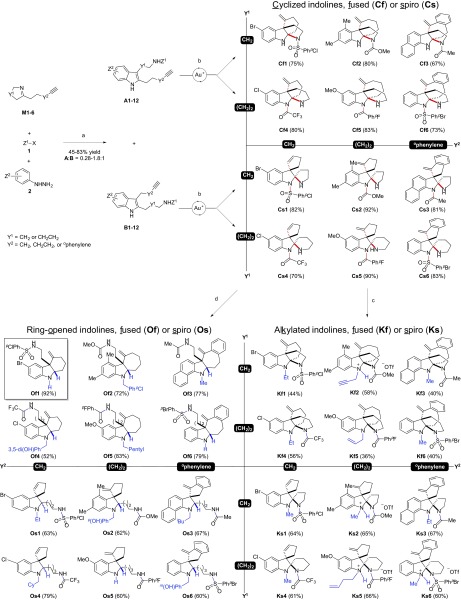
Fig. 3.
Bio-inspired synthesis of polycyclic indolines. Numbers in parentheses are isolated yields. Conditions and reagents: (a) 1, DMAP, 23 °C, 0.5 h, DMF; M, 2–12 h; TsOH⋅H2O, 23–80 °C, 24 h, 45–83%; (b) Ph3PAuNTf2, 50 °C, toluene, 1–12 h; 67–95%; (c) R1OTf, DCM, 23 °C, 2–12 h, 30–92%; (d) AcOH, NaBH3CN, MeOH, 0 °C, 0.5 h, and then aldehyde, 0–23 °C, 2–12 h, 35–99%. DMAP, 4-dimethylaminopyridine; DMF, N,N-dimethylformamide; TsOH·H2O, p-toluenesulfonic acid monohydrate; Ph3PAuNTf2, [bis(trifluoromethanesulfonyl)imidate]-(triphenylphosphine)gold(I); OTf, trifluoromethanesulfonate; DCM, dichloromethane; AcOH, acetic acid, NaBH3CN, sodium cyanoborohydride; MeOH, methanol; TMS, trimethylsilyl; Ph, phenyl; iBu, isobutyl; Cy, cyclohexanyl; Me, methyl; Et, ethyl. Full details are in SI Appendix, SI Methods.