Abstract
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a well-established therapeutic option for hematological malignancies. Combination antiretroviral therapy (cART) has enabled the treatment of medical conditions in patients infected with the human immunodeficiency virus (HIV) in the same way as in the general population. Moreover, improvements in supportive care have allowed HIV-infected patients with life-threatening hematological disorders to be treated with Allo-HSCT. We report on four HIV-infected patients with hematological malignancies receiving an Allo-HSCT in our institution, and on the use of donor lymphocyte infusions to successfully treat post-Allo-HSCT relapse. Of note, one of them is the first HIV+ patient to receive a “dual transplant” (unrelated umbilical cord blood stem cells combined with mobilized T cell-depleted CD34+ stem cells from a mismatched third party donor). cART drugs interactions were satisfactorily managed. This approach provided long-term control of the hematological disease. Nevertheless, despite adequate immune reconstitution, infections were the main cause of morbidity and mortality after Allo-HSCT.
Introduction
Patients with human immunodeficiency virus (HIV) infection receiving combination antiretroviral therapy (cART) have a high survival rate.1 However, HIV-infected patients show a higher risk of developing not only AIDS-defining hematological malignancies, such as non-Hodgkin's lymphoma (NHL), but also other hematological malignancies such as Hodgkin's disease, multiple myeloma, or leukemia.2,3 Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is a well-established option for the treatment of such life-threatening conditions in HIV-negative patients.4 Recent reports have shown that the combination of autologous hematopoietic stem cell transplantation (Auto-HSCT) and cART in HIV-infected patients with associated lymphoma is a feasible and safe approach, with results similar to those observed in HIV-negative patients.5–7 In addition, broad experience with solid organ transplantation in HIV-infected patients has shown this procedure to be both safe and useful, with no significant toxicity due to interactions between cART and immunosuppressive agents.8,9
However, the literature contains few case reports of Allo-HSCT in HIV-infected patients with high-risk hematological malignancies.10–22 An excellent review of reported cases has been recently published by Hutter and Zaia.23 In this context, infections arising from increased immunodeficiency and the potential pharmacological interactions between cART, conditioning treatment, and immunosuppressive drugs are the main concerning issues of this procedure. Furthermore, the impact of the graft-versus-tumor (GVT) effect has yet to be evaluated in HIV-infected patients undergoing Allo-HSCT.
We report on our experience treating four HIV-infected patients with hematological malignancies using cART and Allo-HSCT in our institution. The series includes the first HIV-positive patient to receive umbilical cord blood stem cells (UCB) with the coinfusion of mobilized T-depleted CD34+ stem cells from a mismatched third party donor, known as “dual transplant.”24 Additionally, we also describe the use of donor lymphocyte infusion (DLI) for the treatment of tumor relapse after Allo-HSCT in one patient.
Patients and Results
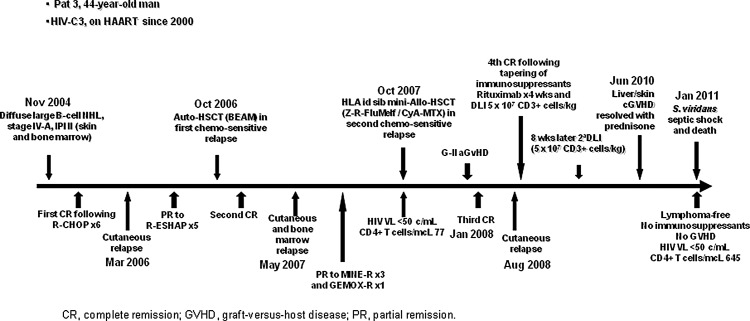
Characteristics of the four patients and of the allo-HSCT performed are detailed in Table 1. Moreover, evolution of patient 3, who received a DLI to treat tumor relapse, is shown in Fig. 1.
Table 1.
Characteristics of the Four Patients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Diagnosis | NHL (DLBCL) IV-B | ALL | NHL (DLBCL) IV-A | NHL (Burkitt L.) IV-B |
| Age (years) | 37 | 45 | 44 | 34 |
| Status at transplant | Chemosensitive relapse | First complete remission | Partial remission | Second complete remission |
| CD4+ HIV-VL at transplant | 54 (cells/μl) <50 copies/ml |
39 (cells/μl) <50 copies/ml |
77 (cells/μl) <50 copies/ml |
73 (cells/μl) 65 copies/ml |
| cART | 3TC, d4T, EFV, NFV | T20, d4T, ABC, 3TC | ddI, T20, 3TC | ABC, 3TC, raltegravir |
| Donor type | Sibling matched | Sibling matched | Sibling matched | Cord blood cell+mismatched third party-related donor |
| Conditioning treatment | MAC TBI+Cy | MAC TBI+Cy | RIC 90YIT+Ritx+Flu+Melf | MAC Flu+Cy+Bu |
| GVHD prophylaxis | CsA | CsA+Mtx | CsA+Mtx | CsA+steroids |
| Neutrophil engraftment, day (d) | Not reached | +17 d | +13 d | +15 d |
| Platelet engraftment, day (d) | Not reached | +14 d | +18 d | +31 d |
| Full donor chimaerism, day (d) | Not reached | +26 d | +32 d | +30 d |
| Acute GVHD grade | NA | III | II | No |
| Chronic GVHD | NA | Extensive | Extensive | No |
| LFU | 6 days | 36 months | 40 months | 9 months |
| Status at LFU | Dead | Dead | Dead | Alive |
ALL, acute lymphoblastic leukemia; cART, combined antiretroviral therapy; CsA, cyclosporine A; DLBCL, diffuse large B cell lymphoma; engraftment day (d) neutrophil >0.5×109/liter and platelets count >20×109/liter; Flu+Cy+Bu, fludarabine, cyclophosphamide, and IV busulfan; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; HIV-VL, HIV viral load; LFU, last follow-up; MAC, myeloablative conditioning; Mtx, methotrexate; NA, not applicable; NHL, non-Hodgkin's lymphoma; RIC, reduced intensity conditioning; TBI, total body irradiation; Cy, cyclophosphamide; 90YIT+Ritx+Flu+Melf, 90Y-ibritumomab-tiuxetan, rituximab, fludarabine, and melphalan; 3TC, lamivudine; d4T, stavudine; EFV, efavirenz; NFV, nelfinavir; T20, enfuvirtide; ABC, abacavir; ddI, didanosine.
FIG. 1.
Graft-versus-tumor effect after allogeneic hematopoietic stem cell transplantation (Allo-HSCT) and donor lymphocyte infusion (DLI) in relapsing HIV lymphoma.
Patient 1
A 37-year-old man was diagnosed with HIV-C3 infection in 1988 and initiated cART in 1996. Two years later he developed diffuse large B cell lymphoma (DLBCL), stage IV-B, with multiple lymphadenopathies as well as lung and bone marrow involvement. He achieved complete remission (CR) with CHOP chemotherapy. He had an early (less than 3 months) skin and bone marrow relapse and received ESHAP as a second line of chemotherapy, followed by an Allo-HSCT from an HLA-identical sibling while in chemosensitive relapse. Total body irradiation (TBI, 4 grays/day for 3 days) plus cyclophosphamide (Cy 50 mg/kg/day for 2 days) were administered as myeloablative conditioning treatment (MAC) and cyclosporine A (CsA) as prophylaxis for graft-versus-host disease (GVHD). Acyclovir, quinolones, cotrimoxazole, and fluconazole were used as antimicrobial prophylaxis. cART was maintained during transplantation. The patient died on day +6 from respiratory distress before engraftment with undetectable HIV viral load (HIV-VL). Autopsy showed pulmonary aspergillosis with no evidence of additional toxicity or lymphoma.
Patient 2
A 45-year-old woman was diagnosed with HIV-C3 infection in 1994, and initiated cART in 1996. Nine years later, she was diagnosed with acute lymphoblastic leukemia, with normal karyotype and BCR-ABL negative as determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization. She achieved CR after chemotherapy (Hyper-CVAD+rituximab). She received an Allo-HSCT from an HLA-identical sibling using TBI+Cy as MAC and CsA+methotrexate (Mtx) as GVHD prophylaxis. Acyclovir, quinolones, cotrimoxazole, and fluconazole were used as antimicrobial prophylaxis. cART was maintained throughout the procedure. Full donor chimerism (FDCh) was observed on day +26. Grade III acute GVHD (a-GVHD) of the skin observed on day +30 was successfully treated with steroids, CsA, and mycophenolate-mofetil. Cytomegalovirus reactivation was observed on day +35 and was successfully treated with gancyclovir. Four months after transplantation the patient developed extensive chronic GVHD (c-GVHD) that was managed with steroids and CsA. She also experienced several episodes of pneumococcal pneumonia and an episode of encephalitis of unknown etiology, despite adequate immunological recovery since month +6 (CD4+ counts: 429 cells/μl; IgG concentration: 683 mg/dl). She started a vaccination program against infection by Streptococcus pneumoniae and Haemophilus influenza in month +7 and received wide antimicrobial prophylaxis with antibiotics and nonspecific immunoglobulins. Despite active therapy, c-GVHD progressed with hepatic dysfunction, ascites, and pleural effusion at month +35, and the patient developed sepsis and Haemophilus spp. pneumonia. This episode was further complicated by a multiresistant Acinetobacter baumannii infection, and the patient died 36 months after transplantation. Throughout the whole posttransplant period (last study month +30), the patient remained in cytological CR with negative minimal residual disease by immunophenotypic bone marrow analysis (less than 0.01% of anomalous cells as determined by four-color flow cytometry) and FDCh, as well as undetectable HIV-VL.
Patient 3
A 44-year-old man was diagnosed with HIV-C3 infection, and initiated cART in 2000. In 2004, he developed DLBCL, stage IVA, with multiple lymphadenopathies and skin and bone marrow involvement. The clinical course and the treatments used before (including an auto-HSCT) and after allo-HSCT are shown in Fig. 1. He achieved CR after six cycles of R-CHOP. However, a new line of chemotherapy (R-ESHAP) had to be administered because of a first skin relapse. Subsequently, he achieved a new CR after consolidation therapy with Auto-HSCT in the first chemosensitive relapse. Seven months after Auto-HSCT a new relapse, with recurrent cutaneous and bone marrow involvement, was diagnosed. The patient received a third line of chemotherapy and an Allo-HSCT from an HLA-identical sibling using reduced intensity conditioning (RIC) with 90Y-ibritumomab-tiuxetan, rituximab, fludarabine, and melphalan. CsA+Mtx were used as GVHD prophylaxis. Acyclovir, quinolones, cotrimoxazole, and itraconazole were used as antimicrobial prophylaxis. cART had to be stopped thereafter for 7 days (days +6 to +12) due to severe mucositis. FDCh was achieved on day +32. The patient developed cytomegalovirus reactivation on day +35, which was successfully treated. A new CR was achieved with bone marrow, skin, and computed tomography (CT) scan free of lymphoma on day +100. Grade II a-GVHD was observed on day +70, initially responding to steroids plus tacrolimus but subsequently progressing to extensive c-GVHD. An episode of meningoencephalitis of unknown etiology was diagnosed on day +90 and appropriately treated. Immune reconstitution at month +8 showed CD4+ counts of 290 cells/μl and an IgG concentration of 559 mg/dl. A new skin lymphoma relapse, with no other tissues affected (negative CT scan and bone marrow without lymphoma infiltration), occurred at month +10. Immunosuppression treatment was tapered followed by 4 weekly doses of rituximab combined with a DLI containing 5×107 CD3+ cells/kg, after which the patient achieved a new CR with the disappearance of skin lesions. In the absence of GVHD manifestations, a second DLI (the same CD3+ cell dose) was given 8 weeks after the first DLI. Extensive c-GVHD (with skin and liver involvement) was diagnosed 22 months after the first DLI that responded adequately to steroids. The patient remained in CR, with no skin infiltration and negative CT scan, 40 months after transplant and 30 months after DLI, with no apparent GVHD and free of immunosuppressive treatment. However, he developed sepsis with multiorgan failure due to Streptococcus viridans infection and died with HIV-VL <50 copies/ml and a CD4+ cell count of 645 cells/μl while receiving cART (Fig. 1).
Patient 4
A 30-year-old man was diagnosed in 2011 with HIV-C3 infection and Burkitt lymphoma, stage IV-B, with multiple lymphadenopathies as well as liver and bone marrow involvement. cART, intensive chemotherapy, and immunotherapy (PETHEMA protocol Spain25) were started, achieving undetectable HIV-VL and CR, respectively. A relapse with an affected bulky retroperitoneal lymphadenopathy occurred 11 months later. He received chemotherapy (R-EDOCH+Mtx/R-IVAC) in combination with involved field radiotherapy, attaining a CR2 (negative PET/CT scan). At this stage, the patient received consolidation therapy with “dual Allo-HSCT” in 2012 using MAC consisting of fludarabine, Cy, and IV busulfan. The UCB unit infused contained 2.56×107 total nucleated cells/kg and 1.3×105 CD34+ cells/kg, while the third party-related donor graft contained 3.46×106 CD34+ cells/kg and 1.1×103 CD3+ cells/kg. HLA identity was 5/6 between the UCB and the patient and 3/10 between the third party donor and the patient. Unfortunately, no eligible HLA-matched unrelated donor was found and a suitable CCR5 Δ32-deleted cord blood unit could not be used. cART was maintained during transplantation, CsA and steroids were used as GVHD prophylaxis, and acyclovir, quinolones, cotrimoxazole, and micafungin were used as antimicrobial prophylaxis. Full cord blood chimerism was detected on day +30. Escherichia coli bacteremia and BK virus-associated hemorrhagic cystitis (grade II) were diagnosed and successfully managed during the first month. No acute GVHD was observed and immunosuppression was withdrawn on day +83. After 9 months, the patient remains in CR (PET/CT scan negative, bone marrow without infiltration, and full donor chimerism) with CD4+ cell counts of 109 cells/μl, with an IgG concentration of 891 mg/dl, and with undetectable HIV-VL while on cART.
Discussion
Allo-HSCT is a well-established approach with curative potential for hematological malignancies in immunocompetent patients.4 Moreover, DLI is an additional immunotherapy option to successfully treat posttransplant relapse.26 Despite the reduced number of reports on HIV-infected Allo-HSCT recipients with hematological malignancies during the cART era,10–22 its therapeutic effect appears to be similar to that observed in HIV-negative patients.
Patients reported here showed no evidence of their original hematological malignancies after Allo-HSCT. Three of them showed long-term control of their hematological disease. Moreover, patient number 3, who experienced NHL relapse after Allo-HSCT, was successfully treated with DLI plus rituximab. In HIV-negative patients, a GVT effect has been observed after using DLI as rescue therapy for lymphoma relapses after Allo-HSCT.27 Although a synergistic effect of the conditioning treatment, and rituximab in the patient receiving DLI, in controlling the primary disease cannot be dismissed, GVT of the donor immune system may have played a role in the long-term control of the hematological disease in the cases reported here (Fig. 1).
The first three patients reported here received peripheral blood stem cells (PBSCs) from HLA-identical sibling donors, with an MAC setting in two patients and an RIC setting in one. Patient 4, however, received a single UCB transplant combined with mobilized T cell-depleted CD34+ stem cells from a mismatched third party donor (“dual transplantation”).24 The aim of this innovative procedure is to reduce the duration of posttransplant neutropenia associated with a single UCB transplant. In fact, this patient constitutes the first report of an HIV-positive patient to receive this combined platform for HSCT. In our experience with HIV-negative patients, “dual transplantation” has shown similar engraftment and survival results as compared to Allo-HSCT from matched unrelated donors.28
Engraftment was successful, with FDCh within the first month posttransplant, in the three evaluable patients (patients 2–4) reported here (Table 1). Moreover, all reported transplant recipients who received PBSC (16) or bone marrow stem cells (2) also showed successful engraftment.10–21 Nevertheless, one patient receiving a single UCB transplantation experienced a primary graft failure, but recovered with a second UCB transplant.22
Allo-HSCT in HIV-infected patients receiving cART requires careful consideration of potential drug interactions.29 All protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) are metabolized in the liver by the cytochrome P450 system (CYP). PIs inhibit the cytochrome P450-3A system, thus increasing levels of immunosuppressive agents and azoles, whereas some NNRTIs induce the P450-3A system. Unlike PIs and NNRTIs, nucleoside reverse transcriptase inhibitors (NRTIs) are not metabolized by the CYP pathway. No clinically significant drug–drug interactions have been identified with enfuvirtide, a peptide fusion inhibitor, or raltegravir, an HIV integrase inhibitor.
We used PIs only in patient 1. More recently NNRTIs, NRTIs, fusion inhibitor, and integrase inhibitor have been used. We did not find adverse effects or significant drug interactions. In fact, cART was maintained and was effective during the whole transplant procedure in all our patients except for patient 3, in which it was transitorily interrupted.
Due to the expected increased risk of opportunistic infections in the context of HIV-infected patients, Allo-HSCT has not been regularly offered to these patients. In fact, our first three patients died of infections. Patient 1 died of invasive aspergillosis in the first week after transplantation. Patients 2 and 3 had several high-risk infections despite adequate immune reconstitution and control of HIV infection. Patient 2 died of Acinetobacter baumannii infection in the context of extensive c-GVHD 36 months after transplant. Patient 3 died of Streptococcus viridans infection 40 months after transplant, although he was free of GVHD and immunosuppressive drugs. However, patient 4 has not had life-threatening infections with a follow-up of 9 months.
The incidence of GVHD reported in HIV-infected transplant recipients appears to be similar to that of HIV-negative recipients. Patients 2 and 3 in this report had both a-GVHD and c-GVHD; nevertheless patient 4 has not developed GVHD 9 months after “dual transplantation.”
Immune reconstitution in the three evaluable cases in the present series, measured as CD4+ cell counts and IgG concentration (mg/dl), does not seem to be different from that reported for HIV-negative patients receiving transplants from both peripheral blood/bone marrow stem cells or cord blood cells.30,31 Similarly, most reported patients reached a CD4+ cell count higher than 200 CD4+ cells/μl 6 to 12 months after transplantation.23
Despite the posttransplant long survival in patients 2 and 3, only patient 4 is currently alive, 9 months after transplantation.
Allo-HSCT is a potential curative approach in HIV-infected patients with hematological diseases, and it could also serve as a platform for the suppression of HIV replication in this patient population. The combination of conditioning treatment, which depletes HIV reservoirs, the inhibition of T cell activation by immunosuppressive agents, and new antiretroviral combinations (including maraviroc, the first CCR5 antagonist of the chemokine receptor for viral entry into CD4+ cells) could reduce the likelihood of reinfection in allotransplanted stem cells. However, HIV-1 latency in resting CD4+ T cells (latent HIV-1 reservoir) makes this procedure insufficient to cure HIV disease. Hutter and Allers21,32 reported the case of a patient who received an Allo-HSCT from an unrelated donor who was homozygous for the infrequent CCR5 Δ32 deletion, which confers high resistance against HIV infection.33 After FDCh, the patient's CCR5 Δ32 heterozygous peripheral blood cells were replaced by homozygous donor cells. RNA and proviral DNA assays were unable to detect HIV-1 virus in the patient despite discontinuation of cART for more than 42 months.
Unfortunately, it is very unlikely that we would find an unrelated HLA-identical adult donor homozygous for the CCR5 Δ32 deletion since it is present in only about 1% of individuals of European descent but it is almost absent in other ethnic groups.34 The search for and selection of homozygous CCR5 Δ32 stem cells could be an interesting strategy in the setting of HIV-positive patients with hematological neoplasms and Allo-HSCT indication. In this regard, umbilical cord blood cell transplantation, not requiring a complete HLA match between recipient and donor, increases the likelihood of finding such a donor.35 We, in fact, searched for a UCB unit with homozygous CCR5 Δ32 deletion for patient 4, but could not find any suitable cord blood unit with such a feature.
Gene therapy aimed at modifying HIV entry coreceptors on the donor's hematopoietic stem cells was explored 10 years ago, but the difficulty of maintaining an adequate proportion of genetically modified stem cells was the main issue.14 Recently, Kiem et al.36 have reviewed the encouraging progress made in this field, with gene-modified hematopoietic stem cells resistant to HIV in the stem cell transplantation setting.
In summary, despite adequate immune reconstitution and control of HIV-VL, infections were the main cause of morbidity and mortality in the present series of patients. Allo-HSCT effectively controlled their hematological malignancy through the conditioning treatment plus the graft-versus-tumor effect. Interactions between immunosuppressive agents and cART could be handled without significant difficulties. Although this procedure is still an experimental strategy with high associated toxicity, Allo-HSCT may be offered as a treatment option for selected HIV-infected patients with high-risk hematological malignancies.
Acknowledgments
The authors thank Thomas O'Boyle for writing assistance during the preparation of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Palella FJ. Delaney KM. Moomam AC. Loveless MO. Fuhrer J. Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Biggar RJ. Chaturvedi AK. Goedert JJ. Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 3.Simard EP. Pfeiffer RM. Engels EA. Spectrum of cancer risk late after AIDS in the United States. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, editor; Forman SJ, editor; Negrin RS, editor; Blume KG, editor. Thomas' Hematopoietic Cell Transplantation. 4th. Wiley-Blackwell; Hoboken, NJ: 2009. [Google Scholar]
- 5.Balsalobre P. Diez-Martin JL. Re A. Michielli M. Ribera JM. Canals C, et al. Autologous stem-cell transplantation in patients with HIV-related lymphoma. J Clin Oncol. 2009;27:2192–2198. doi: 10.1200/JCO.2008.18.2683. [DOI] [PubMed] [Google Scholar]
- 6.Diez-Martin JL. Balsalobre P. Re A. Michielli M. Ribera JM. Canals C, et al. Comparable survival between HIV+and HIV– non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation. Blood. 2009;113:6011–6014. doi: 10.1182/blood-2008-12-195388. [DOI] [PubMed] [Google Scholar]
- 7.Serrano D. Miralles P. Balsalobre P. Diez-Martin JL. Berenguer J. Hematopoietic stem cell transplantation in patients infected with VIH. Curr HIV/AIDS Rep. 2010;7:175–184. doi: 10.1007/s11904-010-0050-8. [DOI] [PubMed] [Google Scholar]
- 8.Qiu J. Terasaki PI. Waki K. Cai J. Gjertson DW. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006;81:1658–1661. doi: 10.1097/01.tp.0000226074.97314.e0. [DOI] [PubMed] [Google Scholar]
- 9.Tateo M. Roque-Afonso AM. Antonini TM. Medja F. Lombes A. Jardel C, et al. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: Perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. AIDS. 2009;23:1069–1076. doi: 10.1097/QAD.0b013e32832c2a37. [DOI] [PubMed] [Google Scholar]
- 10.Schlegel P. Beatty P. Halvorsen R. McCune J. Successful allogeneic bone marrow transplant in an HIV-1-positive man with chronic myelogenous leukemia. J Acquir Immune Defic Syndr. 2000;24:289–290. doi: 10.1097/00126334-200007010-00017. [DOI] [PubMed] [Google Scholar]
- 11.Sora F. Antinori A. Piccirillo N. De Luca A. Chiusolo P. Cingolani A, et al. Highly active antiretroviral therapy and allogeneic CD34(+) peripheral blood progenitor cells transplantation in an HIV/HCV coinfected patient with acute myeloid leukemia. Exp Hematol. 2002;30:279–284. doi: 10.1016/s0301-472x(01)00793-7. [DOI] [PubMed] [Google Scholar]
- 12.Binaghi MA. Lasala MB. Longoni H. Corach D. Kohan A. Peripheral blood stem cell transplantation in a patient with acquired aplastic anemia, HIV infection. 48 ASH Annual Meeting; 2006. Abstract no. 5368. [Google Scholar]
- 13.Bryant A. Milliken S. Successful reduced-intensity conditioning allogeneic HSCT for HIV-related primary effusion lymphoma. Biol Blood Marrow Transplant. 2008;14:601–602. doi: 10.1016/j.bbmt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Kang EM. de Witte M. Malech H. Morgan RA. Phang S. Carter C, et al. Nonmyeloablative conditioning followed by transplantation of genetically modified HLA-matched peripheral blood progenitor cells for hematologic malignancies in patients with acquired immunodeficiency syndrome. Blood. 2002;99:698–701. doi: 10.1182/blood.v99.2.698. [DOI] [PubMed] [Google Scholar]
- 15.Hamadani M. Baiocchi R. Lin T. Blum KA. Ezzone S. Benson DM, et al. Feasibility of allogeneic peripheral blood stem cell transplantation (Allo-SCT) following reduced intensity conditioning (RIC) in HIV+ patients with hematological malignancies. Biol Blood Marrow Transplant. 2009;15 Abstract no. 314. [Google Scholar]
- 16.Shamansky S. Fedotova I. Popkov J. Burya A. Rukavicin O. Allogeneic stem cell transplantation in a patient with acute myeloid leukemia and HIV, hepatitis C infections. Bone Marrow Transplant. 2004;33:S344–345. Abstract no. 1215. [Google Scholar]
- 17.Wolf T. Rickerts V. Staszewski S. Kriener S. Wassmann B. Bug G, et al. First case of successful allogeneic stem cell transplantation in an HIV-patient who acquired severe aplastic anemia. Haematologica. 2007;92:e56–58. doi: 10.3324/haematol.11394. [DOI] [PubMed] [Google Scholar]
- 18.Avettand-Fenoel V. Mahlaoui N. Chaix ML. Milliancourt C. Burgard M. Cavazzana-Calvo M, et al. Failure of bone marrow transplantation to eradicate HIV reservoir despite efficient HAART. AIDS. 2007;21:776–777. doi: 10.1097/QAD.0b013e3280b01836. [DOI] [PubMed] [Google Scholar]
- 19.Woolfrey AE. Malhotra U. Harrington RD. McNevin J. Manley TJ. Riddell SR, et al. Generation of HIV-1-specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112:3484–3487. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polizzotto MN. Skinner M. Cole-Sinclair MF. Opat SS. Spencer A. Avery S. Allo-SCT for hematological malignancies in the setting of HIV. Bone Marrow Transplant. 2010;45:584–586. doi: 10.1038/bmt.2009.168. [DOI] [PubMed] [Google Scholar]
- 21.Hutter G. Nowak D. Mossner M. Ganepola S. Mubig A. Allers K, et al. Long-term control of HIV by CCR5 delta32/delta32 stem cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 22.Tomonari A. Takahashi S. Shimohakamada Y. Ooi J. Takasugi K. Ohno N, et al. Unrelated cord blood transplantation for a human immunodeficiency virus-1-seropositive patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36:261–262. doi: 10.1038/sj.bmt.1705028. [DOI] [PubMed] [Google Scholar]
- 23.Hutter G. Zaia JA. Allogeneic haematopoietic stem cell transplantation in patients with human immunodeficiency virus: The experiences of more than 25 years. Clin Exp Immunol. 2011;163:284–295. doi: 10.1111/j.1365-2249.2010.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández MN. Regidor C. Cabrera R. Garcia-Marco JA. Fores R. Sanjuan I, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31:535–544. doi: 10.1016/s0301-472x(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 25.Oriol A. Ribera JM. Bergua J. Gimenez Mesa E. Grande C. Esteve J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: Comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113:117–125. doi: 10.1002/cncr.23522. [DOI] [PubMed] [Google Scholar]
- 26.Kolb HJ. Schattenberg A. Goldman JM. Hertenstein B. Jacobsen N. Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 27.Russell NH. Byrne JL. Faulkner RD. Gilyead M. Das-Gupta EP. Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:437–441. doi: 10.1038/sj.bmt.1705074. [DOI] [PubMed] [Google Scholar]
- 28.Kwon M. Balsalobre P. Serrano D. Perez-Corral A. Buño I. Anguita J, et al. Single cord blood combined with HLA-mismatched third-party donor cells. Comparable results to matched-unrelated donor transplantation in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2013;19:143–149. doi: 10.1016/j.bbmt.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Dec 1, 2009. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Mar 26;2010 ]. pp. 1–161.www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 30.Heining C. Spyridonidis A. Berhardt E. Schulte-Monting J. Behringer D. Grüllich A, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: A retrospective study including 148 patients. Bone Marrow Transplant. 2007;39:613–622. doi: 10.1038/sj.bmt.1705648. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson CA. Turki AT. McDonough SM. Stevenson KE. Haesook T. Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: Comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2013;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allers K. Hutter G. Hofmann J. Loddenkemper C. Rieger K. Thiel E, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 33.Samson M. Libert F. Doranz BJ. Rucker J. Liesnard C. Farber CM, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 34.Lucotte G. Frequencies of 32 base pair deletion of the (delta 32) allele of the CCR5 HIV-1 co-receptor gene in caucasians: A comparative analysis. Infect Genet Evol. 2002;1:201–205. doi: 10.1016/s1567-1348(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 35.Petz LD. Redei I. Brison Y. Regan D. Kurtzberg J. Shpall E, et al. Hematopoietic cell transplantation with cord blood for cure of HIV infections. Biol Blood Marrow Transplant. 2013;19:393–397. doi: 10.1016/j.bbmt.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiem HP. Jerome KR. Deeks SG. McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10:137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]