Figure 1.
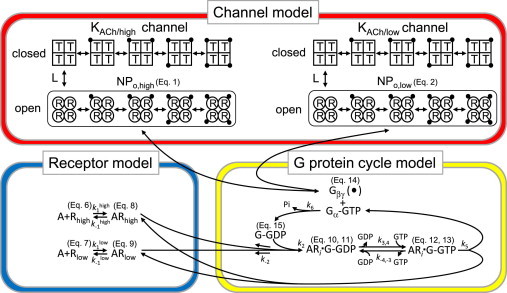
Schematic diagrams of the allosteric model for the KACh channel and the model for receptor-G-protein interaction. Top: Schematic representation of the allosteric model. In this scheme, each KACh channel with either high or low affinities to Gβγ (KACh/high or KACh/low, respectively) is assumed to be an oligomer composed of four identical subunits. Each subunit is in either the available (R, relaxed) or unavailable (T, tense) state, which is represented by a circle or a square, respectively. Each subunit in the R or T state binds to one dissociated G-protein βγ subunit (solid circles) independently of the other subunits, with the microscopic dissociation constants KR or KT, respectively. In this model, all subunits in the same oligomer must change their conformations simultaneously. R4 and T4 are in equilibrium with the allosteric constant L. Bottom: Models of receptor-G-protein interaction. The low-affinity state of m2Rs was incorporated into our previous model (21). A, ACh; Rhigh and Rlow, high-affinity and low-affinity m2Rs, respectively; G, G protein.
