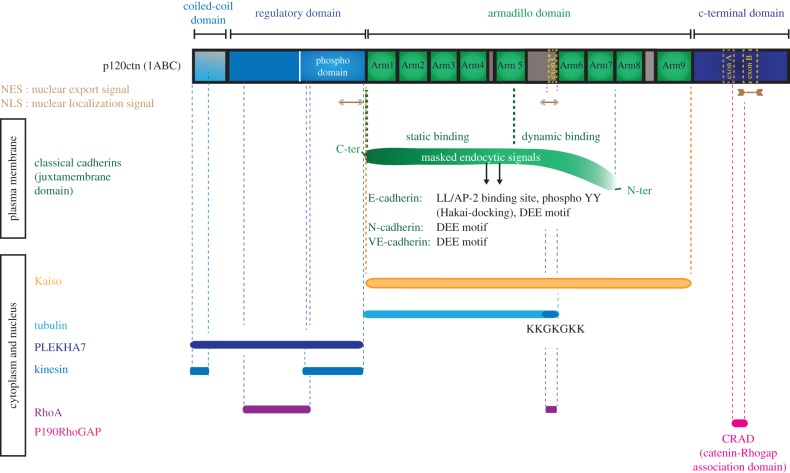
Figure 2.
The main binding partners of p120catenin and their sites of interaction. The main binding partners of p120catenin are listed. A bar of the corresponding colour is positioned above their known binding site on the p120catenin protein. The association of classical cadherins with p120catenin both occurs through a static and a less potent, dynamic binding (indicated by a curve), and is essential to prevent their endocytosis because it masks various endocytic signals (dileucin (LL), di-phosphotyrosines (YpYp) or DEE motif) depending on the classical cadherins. Note the ability of p120catenin to directly bind RhoA and to interact with its GAP, p190RhoGAP. p120catenin could modulate the microtubule cytoskeleton through its direct interaction with tubulin, the heavy chain of the molecular motor kinesin, and PLEKHA7, a protein associated with the microtubule minus-end molecule NEZHA.