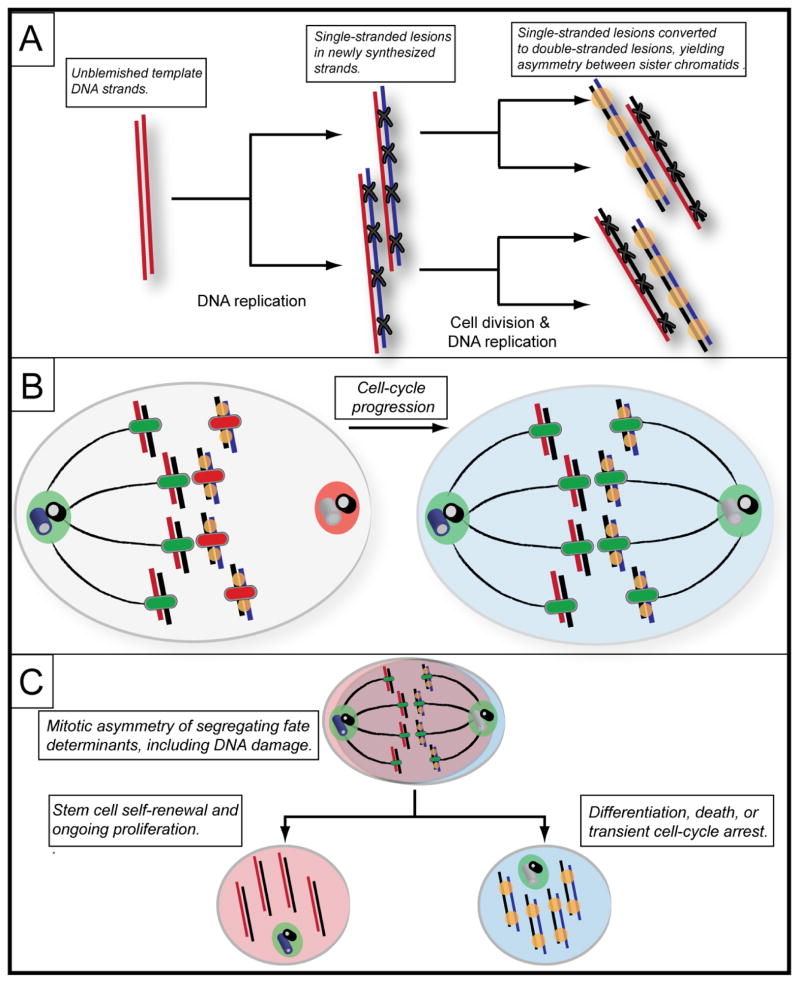
Figure 1. The mortal strand hypothesis.
(A) A chromosome consisting of two unblemished DNA strands (red), each of which serves as a template for synthesis of a new strand (blue) to form two sister chromatids. In our model, because of DNA replication stress, the newly synthesized strand contains lesions (X), such as singlestranded gaps or misincorporated nucleotides. Following sister chromatid separation and cell division, each of the two strands of the chromosome again serves as a template for synthesis of one of two sister chromatids. In this round of DNA synthesis, the replication fork encounters pre-existing, unrepaired lesions on the newer template DNA strand, causing the fork to collapse and form double-stranded breaks (yellow circle). The older, still unblemished template DNA strand again generates a chromatid with single-strand lesions (X) on the newly synthesized strand (black). The resulting sister chromatids are therefore distinguished by the DNA damage each bears. (B) The presence of doublestranded breaks on the sister chromatid containing the newer template strand results in epigenetic changes that are transmitted to the centromere of that chromatid (red centromere). These epigenetic changes transiently inhibit association of the mitotic microtubule spindle with chromatids bearing newer template strands. At the same time, an asymmetry in the spindle-forming capacity of the centrosomes, based on the relative age of the mother centrioles, generates a transient half-spindle. Because the undamaged chromatids bearing older template strands do not contain an inhibitory signal at the centromere (green centromere), the spindle microtubules of the dominant centrosome (green) preferentially attach to these chromatids. Later in the cell cycle, the inhibitory signal at the centromere of the chromatids containing newer template strands is lost, as the non-dominant centrosome forms the second half of the mitotic spindle. The microtubules emanating from the non-dominant “ younger” centrosome therefore form attachments with the chromatids with newer template strands. (C) The non-random segregation of template DNA strands is coordinated with asymmetric fate determination in the two daughter cells resulting from the division. DNA damage, having been segregated asymmetrically, can by itself initiate a signaling pathway that leads to the differentiation or death of the daughter cell inheriting the damage. Another possibility is that fate determinants, such as Numb, are distributed unequally between daughter cells (depicted by the different colors of the cells). The distribution of fate determinants may be coordinated with non-random chromosome segregation, or the two phenomena may occur independently.