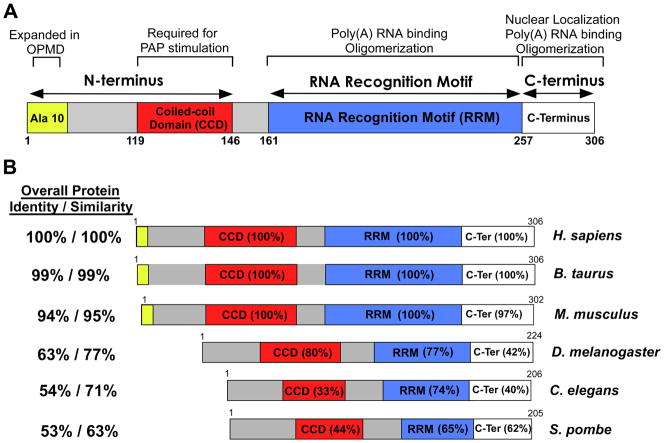
Fig. 1. Domain structures and comparison of nuclear poly(A) binding proteins.
(A) Domain structure of human PABPN1. PABPN1 is made up of three major domains: 1) an N-terminal domain that contains a stretch of 10 alanines that is expanded to between 12 and 17 alanines in autosomal dominant OPMD and a coiled-coiled region required for binding to the poly(A) polymerase (PAP); 2) a single RNA Recognition Motif (RRM) that is required for poly(A) RNA binding and also contributes to oligomerization; and 3) a C-terminal region that contains a nuclear localization signal and contributes to PABPN1 oligomerization. The function(s) attributed to each domain is (are) indicated at the top. (B) Alignment of domain structures of human PABPN1 with the various nuclear poly(A) binding proteins from different organisms that have been studied. Both bovine (B. taurus) and murine (M. musculus) PABPN1 are shown as are nuclear poly(A) binding proteins from D. melanogaster (PABP2), C. elegans (PABP-2), and S. pombe (Pab2). Of the proteins shown, only the human, bovine and mouse PABPN1 proteins contain the N-terminal stretch of alanines that is expanded in OPMD. The Overall Protein Identity/Similarity as compared to human PABPN1 is indicated to the left of each domain structure. The similarity between the domains of different nuclear poly(A) binding proteins and the corresponding domain of human PABPN1 is indicated as percent amino acid identity within each domain.