Figure 1.
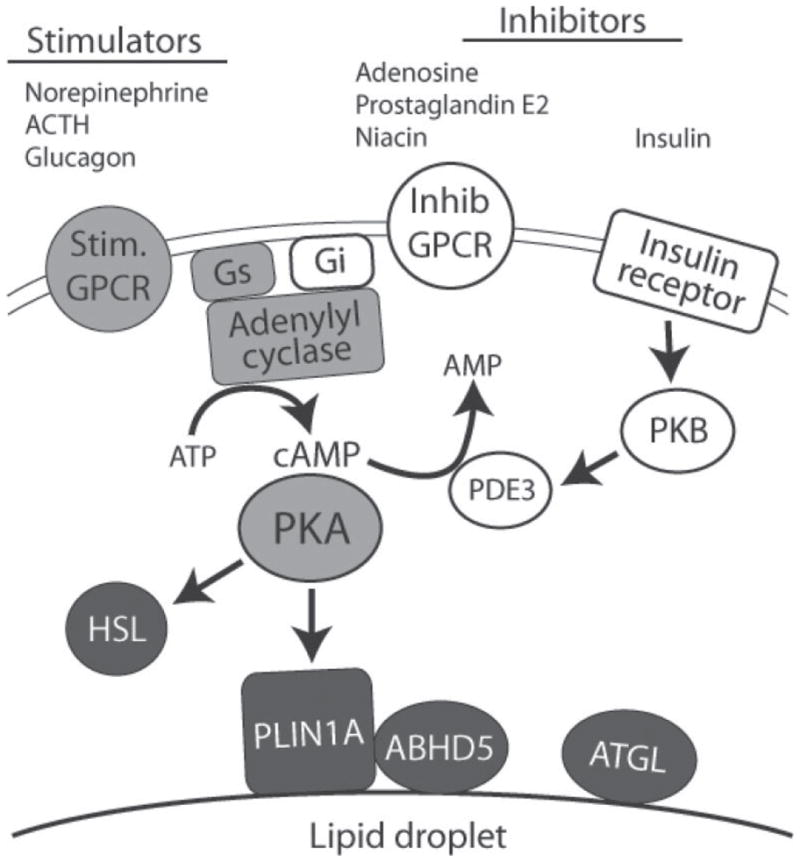
Rapid regulation of adipocyte lipolysis of protein kinase A (PKA). Adipocyte lipolysis is regulated by diverse stimulatory (Stim.) and inhibitory (Inhib) ligands acting through G-protein coupled receptors (GPCR) that transverse the plasma membrane bilayer. G-protein coupled receptor-generated signals are integrated by adenylyl cyclase, which generates cAMP. In addition, insulin suppresses lipolysis through protein kinase B (PKB)-dependent activation of phosphodiesterase 3 (PDE3), which degrades cyclic adenosine monophosphate (cAMP) to adenosine monophosphate (AMP). Signals controlling lipolysis converge at PKA, which directly phosphorylates hormone-sensitive lipase (HSL) and perilipin1A (PLIN1A), the major PKA substrate in fat cells. Perilipin1A is targeted to the surface of lipid droplets that are covered by a phospholipid monolayer. Adipose triglyceride lipase (ATGL) and its co-activator, α/β hydrolase domain-containing 5 (ABHD5), are key elements PKA-regulated lipolysis, yet neither appears to be a direct target of PKA. Gs and Gi are the stimulatory and inhibitory G-proteins, respectively, of adenylyl cyclase.
