Abstract
Background
The absence of coronary artery calcium (CAC) is a marker of very low cardiovascular risk. Endothelial cells may have an effect on the initiation and propagation of arterial calcification. We aimed to identify the relationship between the absence of CAC and endothelial function in individuals without cardiovascular disease and diabetes.
Methods and Results
CAC was assessed using electron-beam computed tomography and the calcium score was then computed. Endothelial function was measured by assessing reactive hyperemia-induced vasodilation and expressed by the reactive hyperemia index (RHI). Of 82 patients, 39 had non-detectable calcium (CAC score=0) and 43 had a CAC score >0. In the CAC score=0 group, the prevalence of normal endothelial function was 84.6%, compared to 48.8% in the CAC score >0 group, P=0.001. The absence of CAC was highly correlated with normal endothelial function (γ=0.704, P<0.001). On average, endothelial function was significantly better in the CAC score=0 group than in the CAC score >0 group (RHI 2.2±0.6 vs. 1.8±0.5, P=0.002). In a multivariate logistic regression model, only normal endothelial function (odds ratio [OR] 5.03, 95% confidence interval [CI] 1.55–16.27, P=0.007) and age (years) (OR 0.91, 95% CI 0.86–0.96, P=0.002) were independently associated with the absence of CAC.
Conclusions
Normal functional status of the vasculature may be important for the prevention of coronary calcification and may partly account for the low cardiovascular risk of absent CAC. (Circ J 2012; 76: 2705 – 2710)
Keywords: Atherosclerosis, Coronary artery calcium, Electron-beam computed tomography, Endothelial function
Vascular calcification plays an important role in the development of atherosclerosis and that partially represents the burden of atherosclerotic plaque.1 The quantification of coronary artery calcium (CAC) can be performed using electron-beam computed tomography (EBCT) and because the presence of CAC gives important prognostic information for subsequent coronary events in individuals with or without cardiovascular disease (CVD),2,3 the technique has been included in current prevention guidelines.4
In recent years, there has been increasing interest in patients who are free from CAC (CAC score=0) on EBCT. Studies with long follow-up have demonstrated dramatically lower cardiovascular (CV) event rates in persons without any detectable CAC. The Multi-Ethnic Study of Atherosclerosis (MESA) showed an event rate of 0.6/1,000 person years among 3,409 patients with no CAC.5 In a cohort of 19,898 asymptomatic middle-age patients free from known coronary artery disease, a CAC score=0 was associated with <1% 10-year risk.6 A metaanalysis involving 64,000 individuals (median follow-up 50 months) showed that the absence of CAC was associated with an annualized event rate of 0.12%.7 Even compared to patients with only a minimal amount of CAC, patients without any calcium still have a statistically significant lower risk of CV events.6 The absence of CAC seems to be a unique entity and a threshold effect on CV risk may exist between the absence and presence of CAC.8,9 Despite the overwhelming evidence linking the absence of CAC and low CV risk, both the mechanism for low CV risk and the threshold effect are unclear.
The endothelium is a highly active organ exerting important homeostatic functions, including the regulation of vascular tone, cell adhesion, smooth muscle cell proliferation, vessel wall inflammation, and it also exhibits antithrombotic properties.10 Endothelial cells also play a crucial role in the initiation, propagation and regulation of artery calcification.11 Previous studies have investigated the association between the CAC score and endothelial dysfunction, but the results have been conflicting. 12–14 The relationship between endothelial function and the absence of CAC has not, to our knowledge, been reported.
The purpose of the current study was to assess the association between endothelial function and the absence of CAC in individuals without CVD or diabetes.
Methods
Study Population
In this retrospective study, 753 patients who underwent an assessment of endothelial function at the Mayo Clinic in Rochester, Minnesota, between the years 2006 and 2010 were identified. We included individuals who had both a CAC scan and evaluation of endothelial function within 1 month. Exclusion criteria included diabetes, known CVD (coronary artery disease, cerebral vascular disease and peripheral artery disease), pregnancy, moderate to severe renal dysfunction, and inflammatory disorders. This study was approved by the Mayo Clinic Institutional Review Board and all individuals gave informed consent.
Overweight status or obesity was defined as body mass index (BMI) ≥25 kg/m2. Coronary artery disease was defined as diameter stenosis ≥50% diagnosed by coronary computed tomography angiography or coronary angiography or as a documented prior myocardial infarction [defined according to the standard definition (serum cardiac biomarker elevation with symptoms of ischemia and/or ECG changes indicative of new ischemia/infarction)]. Cerebral vascular disease and peripheral artery disease were defined according to the prior documented diagnosis in the medical record. Moderate to severe renal dysfunction was defined as estimated glomerular filtration rate <60 ml · min–1 · 1.73 m−2. CV risk was assessed by Framingham risk score.
Demographic, Clinical and Laboratory Characteristics of the Patients
Demographic characteristics (age, sex) and CV risk factors were recorded in all patients. Measurements included resting arterial blood pressure, heart rate, BMI, blood cell count, lipid profile (total, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides), fasting blood glucose and serum creatinine. Blood samples were obtained in the fasting state (at least 12 h).
Peripheral Endothelial Function Measurement
Patients were instructed to fast and to refrain from smoking and strenuous exercise for at least 12 h before measurement. All vasoactive medications were discontinued at least 24 h prior to testing. Peripheral arterial tonometry (PAT) signals were obtained using the EndoPAT 2000 device (Itamar Medical Inc, Caesarea, Israel).
A PAT finger probe was placed on each index finger. Pulsatile volume changes of the distal digit induced pressure alterations in the finger cuff, which were sensed by pressure transducers and transmitted to and recorded by the EndoPAT 2000 device. Endothelial function was measured via a reactive hyperemia (RH)–PAT index (RHI) as previously described.15 The ratio of the PAT signal after cuff release compared to baseline was calculated through a computer algorithm automatically normalizing for baseline signal and indexed to the contralateral control arm. The calculated ratio (RHI) is a validated measure of endothelial function; the higher the RHI the better endothelial function. RHI >1.67 was recommended as a cutoff for normal endothelial function in the user manual of the EndoPAT 2000 device.
EBCT
CAC scanning with EBCT (Imatron; GE Healthcare, Milwaukee, WI, USA) was performed in all patients, each of whom had 40–60 tomograms acquired to cover the entire heart. The field of view was 26 cm with a 512×512 pixel matrix (resolution, 0.51×0.51 mm/pixel). Presence of CAC on EBCT tomograms was defined as 2 or more contiguous pixels with a brightness of at least 130 Hounsfield units. Regions of interest surrounding areas of CAC were traced by trained technicians using custom software.
Statistical Analysis
The normality of all distributions was assessed with the Kolmogorov-Smirnov test. Data summaries were presented as mean ± SD for continuous variables and frequencies, group percentages for discrete variables. Two-sample t-tests, rank sum tests or 1-way analysis of variance (ANOVA) were used to test group differences in the former; the Pearson χ2 test was used for the latter. All tests were 2-tailed and P<0.05 was considered significant. Correlation of CAC and endothelial function was shown by Goodman-Kruskal gamma coefficient. Logistic regression analyses were performed to identify variables with a significant independent association with absence of CAC. JMP 8.0 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
Demographic, Clinical and Laboratory Characteristics of the Patients
A total of 82 individuals were included, with 39 (47.5%) free from CAC (score=0) and 43 (52.5%) with a CAC score >0. Patients in the group without CAC were younger, more often female and had a lower BMI than the group with CAC. The demographic, clinical and laboratory characteristics of the 2 groups are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of the 2 Groups of Patients Assessed for CAC
| CAC score=0 (n=39) | CAC score >0 (n=43) | P value | |
|---|---|---|---|
| Age (years) | 49.0±8.9 | 57.7±10.2 | <0.001 |
| Sex (male %) | 20 (51.3) | 34 (79.1) | 0.01 |
| Hypertension (%) | 8 (20.5) | 14 (32.6) | 0.22 |
| Hyperlipidemia (%) | 17 (43.6) | 20 (46.5) | 0.80 |
| Smoking (%) | 1 (2.6) | 5 (11.6) | 0.12 |
| Overweight and obesity (%) | 24 (61.5) | 35 (81.4) | 0.05 |
| SBP (mmHg) | 119.0±15.7 | 121.4±15.7 | 0.50 |
| DBP (mmHg) | 76.5±10.4 | 78.3±9.9 | 0.43 |
| HR (beats/min) | 71.7±15.8 | 71.2±14.9 | 0.90 |
| BMI (kg/m2) | 26.9±4.8 | 28.8±4.3 | 0.09 |
| Medications | |||
| RAAS inhibitor (%) | 2 (5.1) | 7 (16.3) | 0.53 |
| β-blocker (%) | 10 (25.6) | 11 (25.6) | 0.99 |
| CCB (%) | 4 (10.3) | 7 (16.3) | 0.68 |
| Statin (%) | 12 (27.9) | 10 (25.6) | 0.82 |
| Leukocytes (109/L) | 6.2±1.1 | 6.9±2.5 | 0.09 |
| Glucose (mg/dl) | 100.0±13.9 | 95.1±9.4 | 0.21 |
| Sodium (mmol/L) | 140.2±1.9 | 140.4±1.4 | 0.76 |
| Potassium (mmol/L) | 4.4±0.3 | 4.3±0.3 | 0.37 |
| Creatinine (mg/dl) | 0.95±0.17 | 1.03±0.21 | 0.10 |
| TC (mg/dl) | 208.8±40.9 | 211.5±48.5 | 0.84 |
| TG (mg/dl) | 155.1±151.0 | 163.8±97.0 | 0.82 |
| HDL-C (mg/dl) | 57.8±19.2 | 55.6±12.8 | 0.64 |
| LDL-C (mg/dl) | 126.9±40.9 | 124.7±44.3 | 0.86 |
| hsCRP (mg/L) | 2.1±1.8 | 2.0±1.9 | 0.44 |
| eGFR (ml/min) | 101.5±13.4 | 94.9±18.9 | 0.08 |
BMI, body mass index; CAC, coronary artery calcium; CCB, calcium-channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, serum high-density lipoprotein cholesterol; HR, heart rate; hsCRP, high-sensitivity C-reactive protein; LDL-C, serum low-density lipoprotein cholesterol; RAAS, renin-angiotensin-aldo- sterone system; smoking, current smokers; SBP, systolic blood pressure; TC, serum total cholesterol; TG, serum triglycerides.
CAC and Endothelial Function
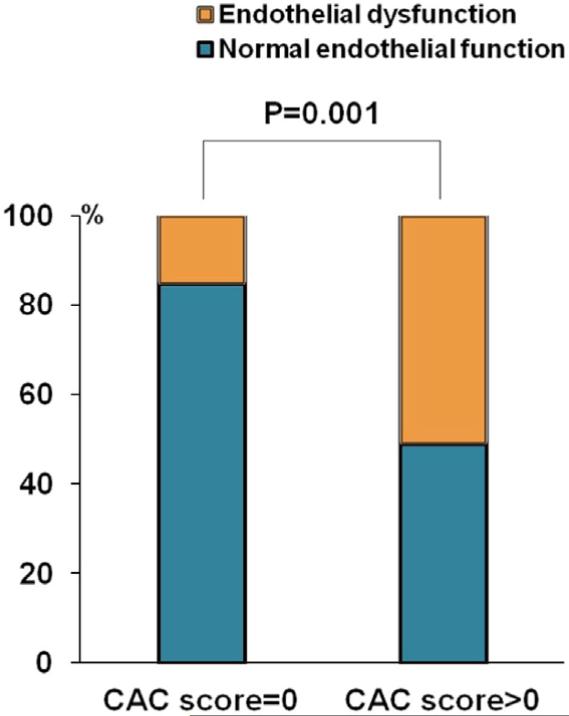
In the CAC score=0 group, the prevalence of normal endothelial function and endothelial dysfunction was 84.6% (n=33) and 15.4% (n=6), respectively, compared to 48.8% (n=21) and 51.2% (n=22) in CAC score >0 group, P=0.001 (Figure 1). The absence of CAC was highly correlated with normal endothelial function (RHI >1.67), γ=0.704, P<0.001. On average, endothelial function was significantly better in the CAC score=0 group (RHI 2.2±0.6 vs. 1.8±0.5, in the CAC score >0 group, P=0.002). The median (Q1, Q3) calcium score was 23.0 (8.7, 107.0) in the CAC score >0 group.
Figure 1.
Absence of coronary artery calcium (CAC) and endothelial function. In CAC score=0 group, the prevalence of normal endothelial function (RHI >1.67) and endothelial dysfunction (RHI ≤1.67) was 84.6% (n=33) and 15.4% (n=6) compared to 48.8% (n=21) and 51.2% (n=21) in CAC score >0 group, P=0.001. RHI, reactive hyperemia index.
10-Year Framingham Risk
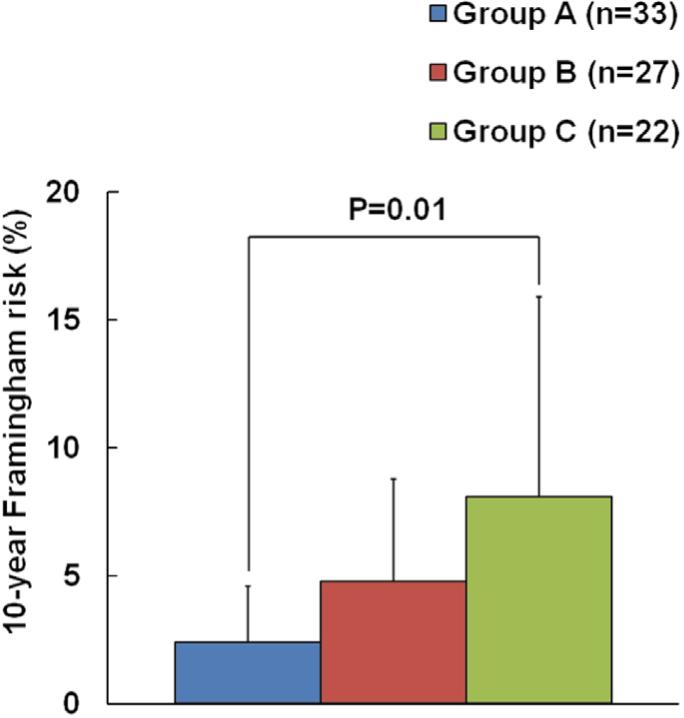
According to endothelial function (RHI) and CAC, we separated all individuals into 3 groups. Group A (n=33): CAC score=0 and normal endothelial function (normal RHI); Group B (n=27): CAC score=0 and endothelial dysfunction (RHI ≤1.67) or CAC score >0 and normal endothelial function; Group C (n=22): CAC score >0 and endothelial dysfunction. The 10-year Framingham risk (%) was 2.4±2.2 in group A, 4.8±4.0 in group B and 8.1±7.8 in group C, P=0.01 (Figure 2).
Figure 2.
The 10-year Framingham risk in 3 subgroups of patients. The risk is lower in individuals with both CAC score=0 and normal endothelial function. Group A: CAC score=0 and normal endothelial function; Group B: CAC score=0 and endothelial dysfunction or CAC score >0 and normal endothelial function; Group C: CAC score >0 and endothelial dysfunction. CAC, coronary artery calcium.
Univariate Analysis
In the univariate analysis, normal RHI, age, sex and BMI ≥25 kg/m2 were significantly associated with the absence of CAC (Table 2).
Table 2.
Determinants of Absent CAC in the Univariate Analysis
| OR | 95% CI | P value | |
|---|---|---|---|
| Normal RHI | 5.76 | 2.00–16.55 | 0.001 |
| Age (years) | 0.91 | 0.86–0.96 | 0.001 |
| Sex (female vs. male) | 3.58 | 1.36–9.40 | 0.008 |
| BMI ≥25 (kg/m2) | 0.33 | 0.13–0.99 | 0.04 |
| SBP | 0.99 | 0.96–1.01 | 0.49 |
| Glucose (mg/dl) | 1.04 | 0.96–1.13 | 0.25 |
| Smoking | 0.20 | 0.02–1.79 | 0.15 |
| LDL-C (mg/dl) | 1.00 | 0.98–1.01 | 0.85 |
| Statins | 0.89 | 0.33–2.37 | 0.81 |
| RAAS inhibitor | 0.27 | 0.05–1.42 | 0.10 |
CI, confidence interval; OR, odds ratio; RHI, reactive hyperemia index. Other abbreviations as in Table 1.
Multivariate Analysis of Determinants for Absent CAC
A multivariate logistic regression model was used to identify determinants of absent CAC. After the inclusion of factors associated with the absence of CAC in the univariate analysis (age, sex, BMI ≥25 kg/m2 and normal endothelial function), only age (years) (odds ratio [OR] 0.91, 95% confidence interval [CI] 0.86–0.96, P=0.002) and normal endothelial function (OR 5.03, 95% CI 1.55–16.27, P=0.007) were independently associated with absence of CAC (Table 3).
Table 3.
Determinants of Absent CAC in a Multivariate Logistic Regression Model
| OR | 95% CI | P value | |
|---|---|---|---|
| Normal RHI | 5.03 | 1.55–16.27 | 0.007 |
| Age (years) | 0.91 | 0.86–0.96 | 0.002 |
| Sex (female vs. male) | 2.56 | 0.65–10.01 | 0.17 |
| BMI ≥25 (kg/m2) | 1.47 | 0.35–6.17 | 0.59 |
| Statins | 0.71 | 0.20–2.55 | 0.60 |
| RAAS inhibitor | 0.18 | 0.03–1.89 | 0.18 |
Discussion
In this study, individuals with both the absence of CAC and normal endothelial function had a lower Framingham risk than individuals who had at least 1 of the 2 risk markers (CAC or endothelial dysfunction). Importantly, we found a strong and independent association between the absence of CAC and normal endothelial function in this non-diabetic population with no CVD, suggesting that normal endothelial function might be an important prerequisite for the absence of CAC.
Endothelial dysfunction is considered to be a surrogate for early atherosclerosis,10 whereas coronary artery calcification develops late in the atherosclerotic process as part of the healing mechanism of usually subclinical plaque rupture events.11 Both are markers of subclinical atherosclerosis and have been shown to be highly predictive for future CV events.2,16 It is intuitive to consider that vascular functional abnormality (endothelial dysfunction) may be closely associated and precede structural damage (the process of calcium precipitation in diseased coronary arteries), similar to the association between carotid intima-media thickness and endothelial function.17 Previous studies have investigated this relationship, but have yielded conflicting results. Whereas Huang et al reported that increased coronary artery calcification strongly predicted endothelial dysfunction (assessed by flow-mediated dilation at brachial artery) in patients with suspected coronary artery disease,18 a recent study from our group found no association between coronary endothelial dysfunction and CAC score in patients with early atherosclerosis.14 It may be that differences in the patient population, the arterial site being tested, and the methods applied produce these inconsistent results. However, 2 studies using the same method (flow-mediated dilation) and involving the same arterial bed (brachial artery) in asymptomatic individuals have also shown different conclusions in assessing the relationship between CAC score and endothelial function.12,13 These inconsistent results may indicate that the association between endothelial dysfunction and CAC is complex. Unlike previous studies that have assessed the relationship between endothelial dysfunction and CAC score, we assessed the relationship between endothelial function and the absence of CAC. As shown in recent clinical studies, the absence of CAC seems to be a unique entity with very low CV risk,5–7 and a threshold effect on CV risk may exist between the absence and presence of CAC (even if a minimal amount of CAC).8 Therefore, the current study has the potential advantage of being more direct and containing greater prognostic implications.
Calcification of the coronary arteries is an active process controlled by complex genetic, cellular and molecular pathways. Normal arteries usually express a number of genes associated with bone formation only at low levels and arterial calcification is actively inhibited.19 During the early stages of atherosclerosis, numerous atherosclerotic and pro-inflammatory factors lead to the expression and activation of osteoblast-like cells, which may start the process of calcium precipitation.20 The initial response in endothelial cells following atherosclerotic and pro-inflammatory stimuli is to release genes that inhibit bone formation (such as matrix gla-protein). Prolonged exposure to these stimuli may lead to expression of genes (such as bone morphogenetic protein-2) that facilitate calcification. 11 We recently demonstrated an increase in osteocalcinexpressing endothelial progenitor cells in patients with endothelial dysfunction.21 These cells were retained within the coronary artery circulation, suggesting that they may play an important functional role in vascular repair.22 Moreover, a recent study demonstrated that calcifying progenitor cells form ectopic calcification in vivo and are overrepresented in atherosclerotic lesions.23 Therefore, endothelial cells could either inhibit or stimulate calcification, depending on the specific background conditions24 and reparative mechanisms.
Normal endothelial function reflects the ability of the endothelium to protect against vascular injury and the initiation of atherosclerosis.25 Because of the close association between the absence of CAC and normal endothelial function in the current study, we speculate that endothelium with normal function may play an important role in inhibiting vascular calcification.
Although it has been reported that statins may be able to inhibit the process of vascular calcification,26 more evidence strongly suggests that pharmacologic therapy and lifestyle modification are lack of obvious effect on arterial calcification,27–29 and no studies have demonstrated that vascular calcification can be reversed. In contrast, endothelial dysfunction is a reversible disorder, and strategies aimed at reducing CV risk factors also translate into an improvement in endothelial health.10 This difference in reversibility between CAC and endothelial dysfunction may be a potential reason for the inconsistent results noted in studies of the association between CAC score and endothelial dysfunction. Given the reversibility of endothelial function, the assessment of endothelial function may serve not only as a tool for the diagnosis of vascular injury and early atherosclerosis, but also as a therapeutic target and a method of following up the success of intervention. A previous study has reported that the improvement in endothelial function was associated with an improvement in CV prognosis and that impairment of endothelial function, despite optimized medical therapy, was a strong independent predictor of adverse CV events.30
The amount of calcium accumulated in any given coronary arterial segment reflects not only the magnitude of the plaque burden, but also the duration of exposure of arteries to the factors that underlie calcification, which renders it largely dependent on age. As in the previous studies,5,6 individuals with advanced age are more likely to have CAC. Although age is closely associated with CAC,31 we have shown that the association between CAC and endothelial function is still independent when correcting for age.
Although many studies have used flow-mediated vasodilatation of the brachial artery, we used the observer-independent PAT technique to measure endothelial function. This FDA-approved technique is commonly used in clinical practice, correlates with coronary microvascular function15 and is prognostic for future cardiovascular events.16
Study Limitations
This was a retrospective, cross-sectional study, where selection bias may occur. However, the prevalence of freedom from CAC and normal endothelial function is similar to results from previous studies, so we believe that our study sample validly represents the population. We applied 1.67 to separate patients with presumably normal and abnormal endothelial function. This value was endorsed by the FDA as a cutoff point of normal and abnormal EndoPAT scores. However, endothelial function is a continuum, so any cutoff value is inevitably somewhat arbitrary. Calcification of the intimal layer of the artery is associated with atherosclerotic plaque and is a predictor of cardiac events, but EBCT cannot distinguish between intimal or medial coronary artery calcification. This may be another explanation for conflicting results in previous studies. Therefore, we excluded individuals with diabetes and moderate to severe renal dysfunction, which may contribute to medial calcification. Finally, our data may not reveal a definite causal relation between endothelial dysfunction and coronary calcification. Nevertheless, the finding has clinical implications and provides helpful information for further research.
Conclusions
In the present study, patients without CAC were significantly more likely to present with normal peripheral endothelial function. This result suggests that the functional status of the endothelium is closely associated with initiation of coronary calcification.
Acknowledgments
Sources of Funding: The investigators’ research is supported by the National Institute of Health (NIH Grant HL-92954 and AG-31750 to A.L., and NIH Grants DK-73608, HL-77131, and HL-085307 to L.O.L.). J.L. received a scholarship from the China Scholarship Council (No. 2010811095) and was supported by the “Beijing Nova program” of Beijing Municipal Science & Technology Commission (A2007079), Beijing China. A.J.F. was supported by the Walter and Gertrud Siegenthaler Foundation, the Young Academics Support Committee of the University of Zurich, and the Swiss Foundation for Medical-Biological Scholarships (SSMBS; SNSF No. PASMP3_132551).
Footnotes
Disclosures
A.L. is a member of the Itamar medical advisory board. The other authors report no potential conflicts of interest and have nothing to disclose.
References
- 1.Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001;26:239–244. doi: 10.1007/pl00002026. [DOI] [PubMed] [Google Scholar]
- 2.Arad Y, Spadaro LA, Goodman K, Lledo-Perez A, Sherman S, Lerner G, et al. Predictive value of electron beam computed tomography of the coronary arteries. 19-month follow-up of 1173 asymptomatic subjects. Circulation. 1996;93:1951–1953. doi: 10.1161/01.cir.93.11.1951. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Ohashi N, Ishibashi K, Utsunomiya H, Kunita E, Oka T, et al. Coronary calcium score as a predictor for coronary artery disease and cardiac events in Japanese high-risk patients. Circ J. 2011;75:2424–2431. doi: 10.1253/circj.cj-11-0087. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 6.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Bonow RO. How low-risk is a coronary calcium score of zero? The importance of conditional probability. Circulation. 2008;117:1627–1629. doi: 10.1161/CIRCULATIONAHA.108.767665. [DOI] [PubMed] [Google Scholar]
- 9.Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK. Presence and severity of noncalcified coronary plaque on 64- slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol. 2007;99:1183–1186. doi: 10.1016/j.amjcard.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 11.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 2004;320:424–427. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]
- 12.Ramadan MM, Mahfouz EM, Gomaa GF, El-Diasty TA, Alldawi L, Ikrar T, et al. Evaluation of coronary calcium score by multidetector computed tomography in relation to endothelial function and inflammatory markers in asymptomatic individuals. Circ J. 2008;72:778–785. doi: 10.1253/circj.72.778. [DOI] [PubMed] [Google Scholar]
- 13.Kullo IJ, Malik AR, Bielak LF, Sheedy PF, 2nd, Turner ST, Peyser PA. Brachial artery diameter and vasodilator response to nitroglycerine, but not flow-mediated dilatation, are associated with the presence and quantity of coronary artery calcium in asymptomatic adults. Clin Sci (Lond) 2007;112:175–182. doi: 10.1042/CS20060131. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Gerber TC, Suwaidi JA, Eeckhout E, Lennon R, Rubinshtein R, et al. Relationship between coronary endothelial function and coronary calcification in early atherosclerosis. Atherosclerosis. 2010;209:197–200. doi: 10.1016/j.atherosclerosis.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 17.Rossi R, Nuzzo A, Olaru AI, Origliani G, Modena MG. Endothelial function affects early carotid atherosclerosis progression in hypertensive postmenopausal women. J Hypertens. 2011;29:1136–1144. doi: 10.1097/HJH.0b013e328345d950. [DOI] [PubMed] [Google Scholar]
- 18.Huang PH, Chen LC, Leu HB, Ding PY, Chen JW, Wu TC, et al. Enhanced coronary calcification determined by electron beam CT is strongly related to endothelial dysfunction in patients with suspected coronary artery disease. Chest. 2005;128:810–815. doi: 10.1378/chest.128.2.810. [DOI] [PubMed] [Google Scholar]
- 19.Guzman RJ. Clinical, cellular, and molecular aspects of arterial calcification. J Vasc Surg. 2007;45(Suppl A):A57–A63. doi: 10.1016/j.jvs.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty TM, Fitzpatrick LA, Shaheen A, Rajavashisth TB, Detrano RC. Genetic determinants of arterial calcification associated with atherosclerosis. Mayo Clin Proc. 2004;79:197–210. doi: 10.4065/79.2.197. [DOI] [PubMed] [Google Scholar]
- 21.Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossl M, Modder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909–2914. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Vigili de Kreutzenberg S, Agostini C, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108:1112–1121. doi: 10.1161/CIRCRESAHA.110.234088. [DOI] [PubMed] [Google Scholar]
- 24.Shin V, Zebboudj AF, Bostrom K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J Vasc Res. 2004;41:193–201. doi: 10.1159/000077394. [DOI] [PubMed] [Google Scholar]
- 25.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 27.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 28.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: A multicenter, randomized, doubleblind trial. Circulation. 2006;113:427–437. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- 29.Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J. 2012;76:176–183. doi: 10.1253/circj.cj-11-0730. [DOI] [PubMed] [Google Scholar]
- 30.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]