Figure 1. Structural descriptions of Jak signaling in the Jak-STAT pathway.
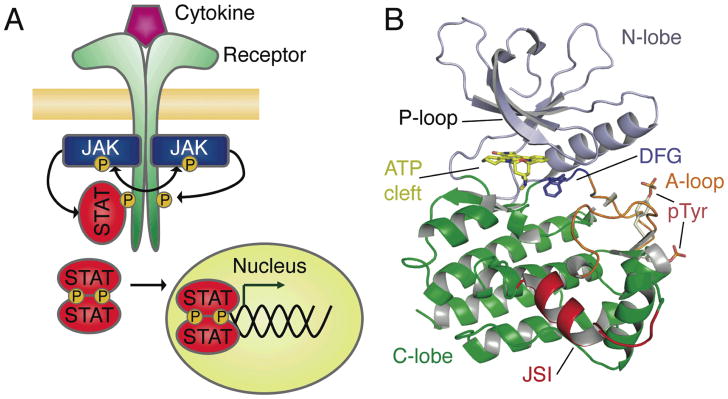
A) The Jak-STAT signaling pathway. Cytokine signaling is initiated upon ligand binding to receptor, this facilitates Jak kinase activation by trans-autophosphorylation. Jaks then phosphorylate the receptor tails creating docking sites for STAT molecules, which bind through their SH2 domains. STATs become phosphorylated by Jaks and form dimers that are able to translocate to the nucleus and regulate gene expression. B) Crystal structures of all Jak kinase domains have been determined in the active state. As is typical for kinase domains, these structures contain a predominantly β-sheet N-lobe (blue) that is conformationally flexible, facilitating activation regulation and ATP/ADP binding and release. The nucleotide-binding glycine-rich loop (P-loop) is indicated. The activation loop (orange) is extended in all of these structures, and is phosphorylated in almost all. The DFG is found in its ‘in’ conformation in all of these structures. The C-lobe of the kinase domain (green) is the most structurally consistent region, with the exception of the Jak-specific insert (red). All structural figures created with Pymol (www.pymol.org).
