Box 1.
Drug summary.
| Drug | Qsymia® (phentermine and topiramate extended-release) |
| Phase | Trials complete for use as a weight-loss therapy; EQUATE (NCT00563368), EQUIP (NCT00554216), CONQUER (NCT00553787), SEQUEL (NCT00796367) |
| Indication | July 2012: FDA approved as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial BMI of 30 kg/m2 or greater, or 27 kg/m2 or greater in the presence of at least 1 weight-related co-morbidity such as hypertension, T2DM or dyslipidemia |
| Pharmacology description/mechanism of action | Phentermine C10H15N · HCl α,α-dimethylphenethylamine hydrochloride. Phentermine hydrochloride is a sympathomimetic amine anorectic agent. The primary mechanism of action of phentermine in treating obesity is postulated to be appetite suppression; however, other CNS actions, or metabolic effects, may also be involved. Topiramate C12H21NO8S 2,3:4,5-di-O-isopropylidene-β-d-fructopyranose sulfamate. Topiramate is a sulfamate-substituted monosaccharide related to fructose used as an antiepileptic agent and for the prophylaxis of migraine headaches. The mechanism of action of topiramate leading to weight loss is also not fully understood, but evidence suggests an effect on energy-balance regulation through a combination of pharmacologic effects, including augmenting the activity of gamma-aminobutyrate, modulation of voltage-gated ion channels, inhibition of AMPA/kainite excitatory glutamate receptors or carbonic-anhydrase inhibition |
| Route of administration | Oral |
| Chemical structure |
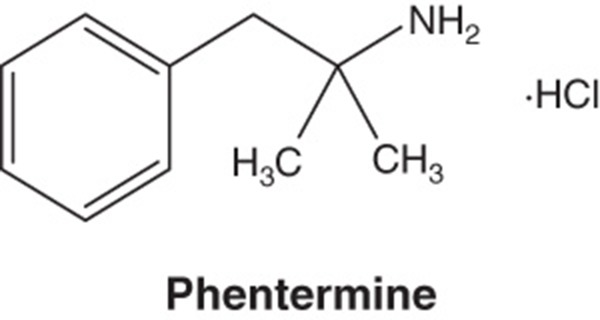
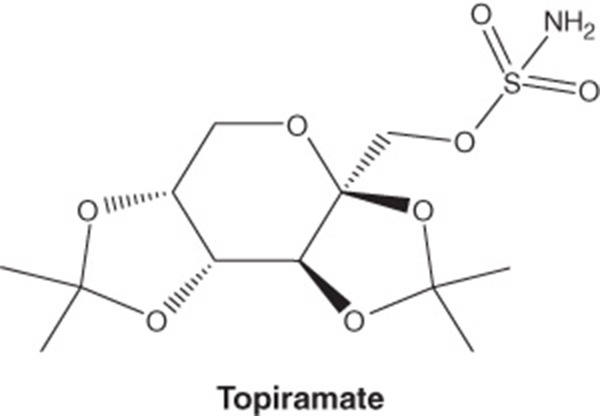
|
| Pivotal trial(s) | EQUIP (NCT00554216); CONQUER (NCT00553787) |
BMI: Body mass index; CNS: Central nervous system; T2DM: Type 2 diabetes mellitus.
