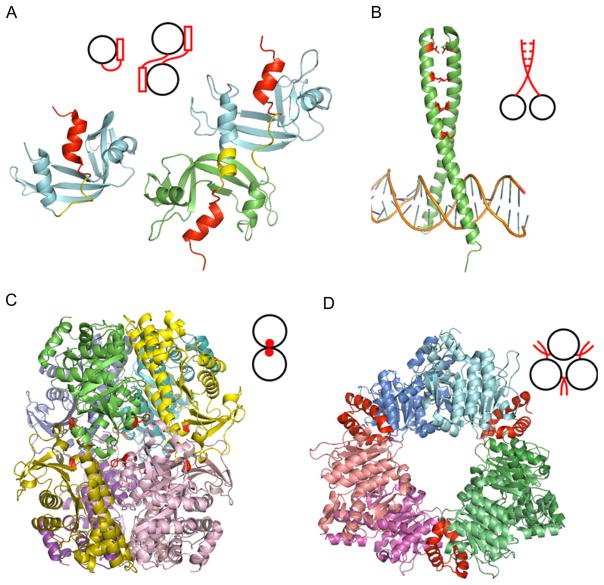
Figure 1.3.
Different mechanisms of homooligomerization. (A) Domain swapping of RNase A: monomer (1RTB) and domain-swapped dimer (1A2W) of RNase A. The swapped regions and domain linker regions are shown as red and yellow, respectively. (B) Leu-zipper of GCN4 (1YSA). Leu at position “d” is shown as stick model and colored in red. (C) Amino acid substitutions for designed homooctamer of L-rhamnulose-1-phosphatase aldolase (2UYU). The original protein exists as a tetramer which is shifted to the octamer upon a single substitution (A88F, shown in red). (D) Insertions of N-acetyl-L-glutamate kinase hexamer (2BUF). Inserted N-terminal helix shown in red enables the hexamer formation. Adapted from Ref. 3.