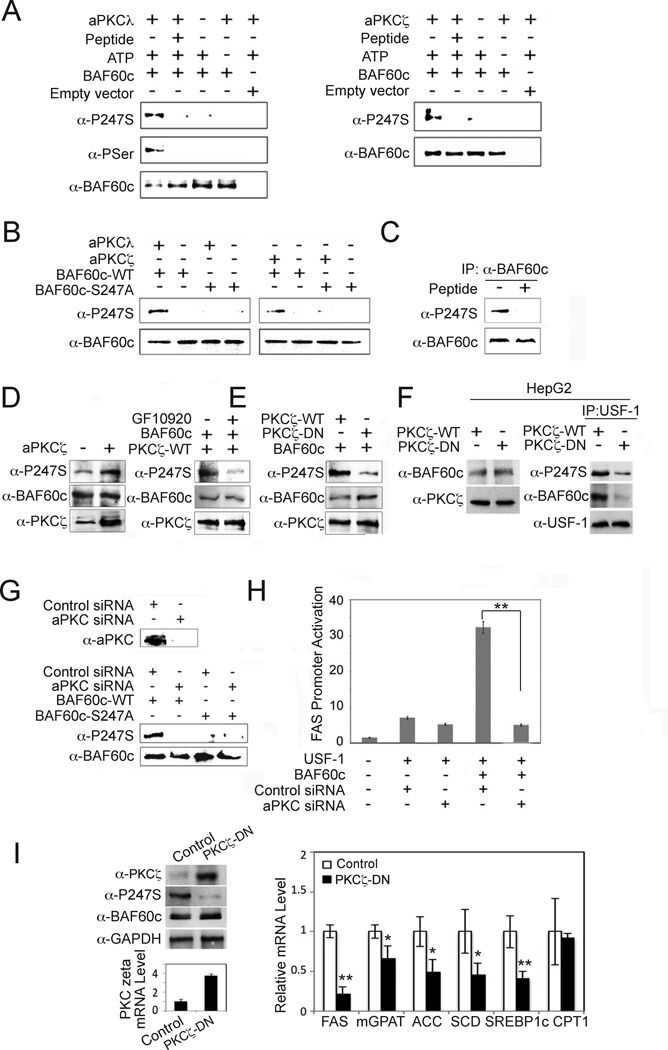
Figure 4. BAF60c is phosphorylated at S247 by aPKC.
(A) In vitro translated BAF60c-HA was in vitro phosphorylated with aPKCλ (left) and ζ (right) in the presence or absence of inhibitory peptide (100 mM) before immunoblotting. (B) In vitro translated wild type or S247A mutant BAF60c were in vitro phosphorylated before immunoblotting. (C) BAF60c-HA transfected cells were incubated with or without aPKC inhibitory peptide. (D) Lysates from 293 cells transfected with BAF60c, with or without PKCζ (left), PKCζ transfected cells treated with GF10920 at 5 mM or control (DMSO) for 30 min (right). (E) Immunoblotting of 293 cells cotransfected with BAF60c along with wild type or dominant negative PKCζ. (F) Lysates from HepG2 cells infected with adenovirus for wild type PKCζ or dominant negative PKCζ (PKCζ-DN) overexpression (left) and after IP with USF-1 antibody (right) before immunoblotting. (G) aPKC protein levels in cells transfected with aPKCζ and aPKCλ siRNA (aPKC siRNA) (top). Immunoblotting of lysates from 293 cells cotransfected with control or aPKC siRNA along with BAF60c or S247A mutant (bottom). (H) FAS promoter activity. Means± SEM. **p<0.01. (I) Immunoblotting of liver lysates from mice after administration of PKCζ-DN (left). Expression of lipogenic genes (right), Means ± SEM. *p<0.05, **p<0.01, n=3–4.