SUMMARY
Despite the prevalence of obesity and its related diseases, the signaling pathways for appetite control and satiety are not clearly understood. Here we report C. elegans quiescence behavior, a cessation of food intake and movement that is possibly a result of satiety. C. elegans quiescence shares several characteristics of satiety in mammals. It is induced by high-quality food, it requires nutritional signals from the intestine, and it depends on prior feeding history: fasting enhances quiescence after refeeding. During refeeding after fasting, quiescence is evoked, causing gradual inhibition of food intake and movement, mimicking the behavioral sequence of satiety in mammals. Based on these similarities, we propose that quiescence results from satiety. This hypothesized satiety-induced quiescence is regulated by peptide signals such as insulin and TGF-β. The EGL-4 cGMP-dependent protein kinase functions downstream of insulin and TGF-β in sensory neurons including ASI to control quiescence in response to food intake.
INTRODUCTION
Uncontrolled appetite and subsequent overeating contribute to obesity and related diseases, but despite the high prevalence of obesity and related diseases, the detailed signaling pathways controlling appetite are not clearly understood. We chose to use Caenorhabditis elegans to study this complex behavior, appetite control, because C. elegans has been used as a powerful genetic model system to understand molecular mechanisms of behavior and has conserved mechanisms of fat storage and energy expenditure (Ashrafi et al., 2003).
In mammals, appetite is controlled by satiety signals from the gastrointestinal tract and adiposity signals from adipose tissue (Schwartz et al., 2000). These two signals are integrated in the hypothalamus to control feeding. Satiety signals result in a specific sequence of behaviors: termination of meals, reduction of locomotion, and rest or sleep (Antin et al., 1975; Gibbs et al., 1973). Because factors other than satiety can cause meal termination, the whole behavioral sequence of satiety is used as an indication of satiation, especially for developing and testing drugs that control appetite (Halford et al., 1998).
C. elegans varies its feeding rate by regulating the rate of pumping, a motion of the pharynx. Feeding rates are high in the presence of food and low in the absence of food (Avery and Horvitz, 1990; You et al., 2006). However, the impact on feeding of the animal’s nutritional status and prior feeding history, factors that influence feeding and satiety in mammals, has not previously been assessed. Under normal laboratory conditions, worms feed and move constantly. In this study, however, we show that under certain conditions, worms stop feeding and moving and become quiescent. Because this quiescence resembles the behavioral characteristics of satiety in other animals and is regulated by molecules such as insulin that are known to control food intake in mammals, we suggest that quiescence is a satiety behavior of worms. Furthermore, we find that the behavior is regulated by cGMP and TGF-β pathways whose functions in appetite control and metabolism have not been studied in mammals.
RESULTS
High-Quality Food Induces Quiescence
When we grew worms on high-quality food (either E. coli HB101 or Comamonas sp.), approximately 90% of the worms were found to be quiescent (Figure 1A), defined as complete cessation of both feeding and moving (see Experimental Procedures and Movie S1 available online). We operationally define “food quality” as the ability to support growth: high-quality food supports growth better than low-quality food (Shtonda and Avery, 2006). In contrast, less than 5% of worms grown on lower-quality food (E. coli DA837) were found to be quiescent (Figure 1A). This suggests that high-quality food induces behavioral quiescence, i.e., termination of feeding and cessation of locomotion. This difference in behavior results from a relatively small difference in food quality—the growth rate on DA837 is 92% of that on HB101 or Comamonas (Shtonda and Avery, 2006). DA837 is derived from OP50, the E. coli strain used routinely by most C. elegans labs to culture worms, suggesting that DA837 does not prevent quiescence by making worms starved or sick.
Figure 1. Quiescence Is Induced by High-Quality Food and Is Dependent on Nutritional Status of Animals.
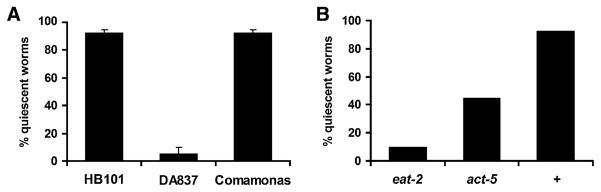
(A) Quiescence (shown as percent quiescent worms) is dependent on food quality. Values are the mean ± SEM of two independent experiments. In each experiment, 20 wild-type worms were tested for each type of food.
(B) Quiescence is dependent on nutrition, as shown by the effect of eat-2 and act-5 mutations. Twenty wild-type worms (+) and 20 of each type of mutant were tested on HB101.
Quiescence Requires Nutritional Signals
Because quiescence is regulated by food quality, we hypothesized that it is dependent on the animal’s nutritional status. To disturb the nutritional status of the animal, we used mutants that either cannot feed well or cannot absorb nutrients well. First, we tested eat-2 mutants, which pump at approximately 15% of the wild-type rate (Avery, 1993; Raizen et al., 1995). On high-quality food, only 10% of eat-2 mutants were quiescent, compared to 90% of wild-type worms (Figure 1B). Second, we tested an act-5 mutant. act-5 encodes a microvillus-specific actin required for absorbing nutrients from the intestine (MacQueen et al., 2005). Approximately 45% of act-5 mutants were quiescent (Figure 1B). This attenuation of quiescence by malnutrition suggests that quiescence depends on nutritional status. The attenuation of quiescence in act-5 mutants also suggests that quiescence requires nutritional signals from the intestine.
Fasting Enhances Quiescence after Full Refeeding
To manipulate the nutritional status of wild-type animals, we fasted young adults for 12 hr, refed them, and compared their quiescence behavior to that of worms that had not experienced fasting. After 3 hr of refeeding, fasted and refed worms typically showed 2- to 4-fold longer quiescence duration than nonfasted worms (Figure 2A). This suggested that fasting and refeeding enhance quiescence. To examine whether the threshold of quiescence was also increased by fasting and refeeding, we disturbed quiescent worms by touching their noses and then measured the probability for the worms to return to quiescence (Movie S2). 100% of the fasted and refed worms returned to quiescence, while only 30% of the nonfasted worms did (Figure 2B). To test whether fasting and refeeding could induce quiescence even on low-quality food, we refed worms with DA837. Worms fasted and refed with DA837 showed more quiescence than worms fed with DA837 without fasting (Figure 2C), indicating that fasting indeed enhanced quiescence. However, induction of quiescence by low-quality food lagged induction of quiescence by high-quality food (Figure 2D), suggesting that it took longer for worms to become quiescent on low-quality food. Also, the level of quiescence with low-quality food did not reach the level of quiescence with high-quality food, suggesting that quiescence after fasting and refeeding also depends on food quality. Our results from the malnutrition mutants and the fasted wild-type worms show that quiescence is regulated by nutritional status as well as feeding history.
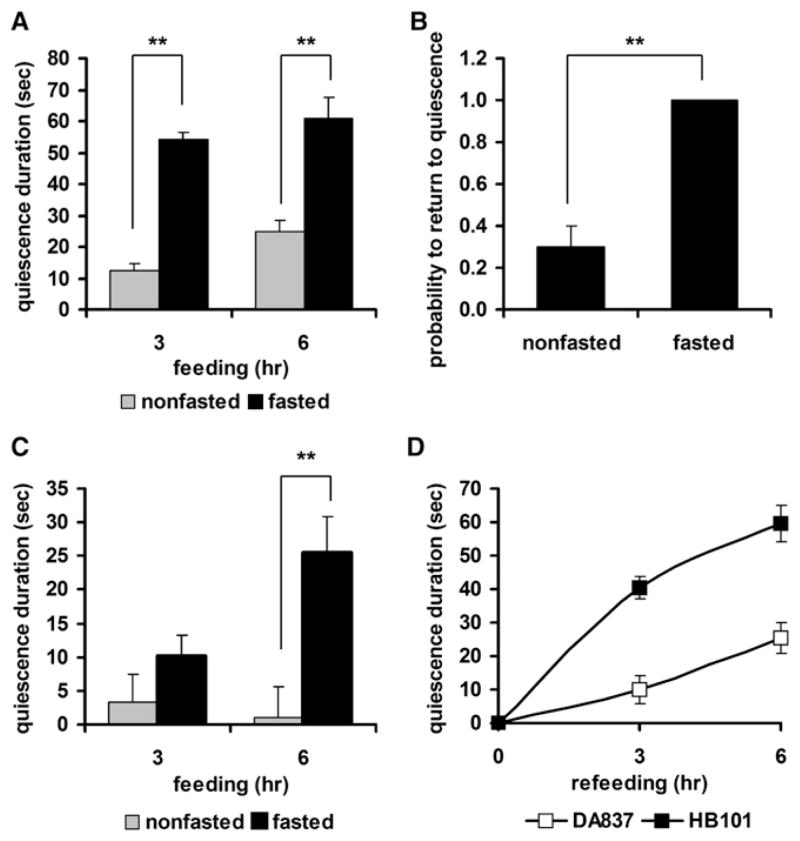
Figure 2. Enhancement of Quiescence by Fasting and Refeeding.
(A) Quiescence duration of fasted and refed worms is longer than that of non-fasted worms. Duration of quiescence was compared for fasted (black bars, n = 13) and nonfasted (gray bars, n = 19) groups after 3 or 6 hr of feeding with HB101.
(B) Fasted and refed worms have a higher probability to return to quiescence than nonfasted worms. The probability to return to quiescence after nose touch (see Experimental Procedures) was compared between fasted (n = 10) and nonfasted groups (n = 11).
(C) Fasting can induce quiescence on low-quality food. Duration of quiescence was compared for fasted (black bars, n = 20) and nonfasted (gray bars, n = 20) groups after 3 and 6 hr of feeding with DA837.
(D) Induction of quiescence by low-quality food lags induction of quiescence by high-quality food. Duration of quiescence was compared for worms refed with HB101 (■) and worms refed with DA837 (□) after 3 or 6 hr of refeeding.
Values are the mean ± SEM. **p < 0.001.
Fasting Enhances Food Intake
To examine how fasting affects refeeding, we compared feeding rates of fasted worms and nonfasted worms. We quantified food intake during the first 5 min of refeeding by measuring fluorescence intensity in the intestine of worms fed GFP-expressing bacteria (see Experimental Procedures). Fasted worms (Figures 3A–3C) fed more than nonfasted worms (Figures 3D–3F). We obtained identical results when we refed worms with fluorescent beads mixed with food (data not shown). As an alternative, we added the fluorescent dye BODIPY to the food. Ingested BODIPY accumulates in the worm, and its fluorescence can be measured (Ashrafi et al., 2003). Because the BODIPY dye needs time to accumulate, we measured fluorescence after 15 min of feeding on food with BODIPY. The fluorescence intensity of the fasted worms was more than 30-fold that of the nonfasted worms, confirming that fasted worms consumed more food than nonfasted worms (Figures 3I and 3J; Figure S1).
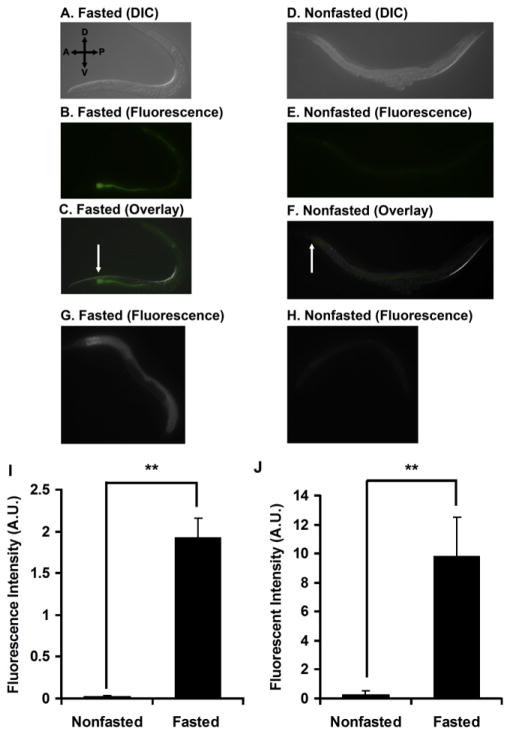
Figure 3. Fasting Enhances Food Intake during the Initial Period of Refeeding.
(A) Differential interference contrast (DIC) image of a worm fasted for 12 hr and refed for 5 min on GFP-expressing HB101.
(B) Fluorescence image of the worm in (A).
(C) Overlay of (A) and (B). The arrow indicates strong GFP signal in the proximal intestine.
(D) DIC image of a nonfasted worm fed for 5 min on GFP-expressing HB101. (E) Fluorescence image of the worm in (D).
(F) Overlay of (D) and (E). The arrow indicates weak GFP signal in the proximal intestine.
(G) Fluorescence image of a worm fasted for 12 hr and refed for 15 min on HB101 mixed with BODIPY dye.
(H) Fluorescence image of a nonfasted worm fed for 15 min on HB101 mixed with BODIPY dye.
(I) Quantification of images from ten fasted and ten nonfasted worms fed on GFP-expressing HB101, expressed in arbitrary units (A.U.) of fluorescence. Data are from one of three independent experiments.
(J) Quantification of images from five fasted and five nonfasted worms fed for 15 min on HB101 mixed with BODIPY dye, expressed in arbitrary units (A.U.) of fluorescence. Data are from one of three independent experiments.
Values are the mean ± SEM. **p < 0.001.
Quiescence Resembles the Behavioral Sequence of Satiety
To examine how quiescence is induced, we measured feeding rates every 5 min during the first 1 hr of refeeding. The initial high feeding rate gradually decreased, and after 1 hr it reached 50% of the initial feeding rate (Figure 4A). During the same refeeding period, food intake measured by fluorescence intensity in the intestines of worms fed GFP-expressing bacteria also gradually decreased (Figure 4B).
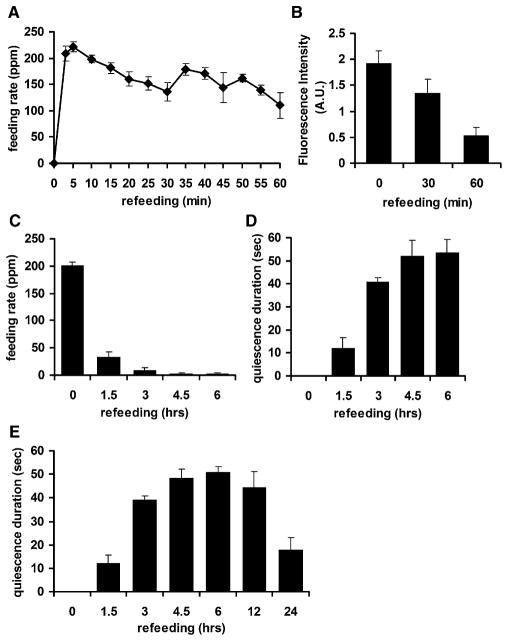
Figure 4. Quiescence Resembles the Behavioral Sequences of Satiety in Mammals.
(A) Decrease in feeding rate (measured in pumps per minute [ppm]) over the time course of refeeding. Feeding rates of each of five wild-type worms were measured every 5 min for the first hour of refeeding.
(B) Decrease in food intake as measured by GFP intensity.
(C) Decrease in feeding rate (measured in ppm) over the time course of refeeding after 12 hr of fasting. Feeding rates of each of 20 wild-type worms were measured at each of the indicated time points (0, immediately after worms were transferred to food; 1.5, 1.5 hr after transfer, etc.)
(D) Increase in quiescence duration over the time course of refeeding. The quiescence durations of ten worms were measured at the indicated time points after transfer to food.
(E) Recovery of normal activity begins after 12 hr of refeeding. The x axis is not linear. Some points are replotted from (D).
Values are the mean ± SEM.
In rodents, fasting and refeeding evoke a behavioral sequence of satiety: gradual attenuation of feeding and locomotion (Antin et al., 1975; Gibbs et al., 1973). When we observed worms every 1.5 hr during refeeding, feeding (as measured by pumping rate) gradually decreased and quiescence (as measured by duration) increased (Figures 4C and 4D). This suggests that quiescence resembles the behavioral sequence of satiety in mammals. The behavior was most consistent between 3 hr of refeeding, when 95.4% of the worms (767 out of 804) were quiescent, and 6 hr of refeeding, when 99.3% of the worms (798 out of 804) were quiescent (Table S1). After 6 hr of refeeding, the behavior became less consistent (Figure 4E). For technical reasons (see Experimental Procedures for details), we were unable to track individual worms throughout the entire refeeding time course. However, since 95% of worms observed after 3 hr of refeeding were quiescent, we deduce that, on the average, an individual worm must spend 95% of its time quiescent at this point. In summary, quiescence can be induced by high-quality food and is attenuated by malnutrition. Moreover, the behavioral sequence (attenuation of feeding and locomotion) leading to quiescence is similar to that of satiety in mammals, suggesting that quiescence is a result of satiety.
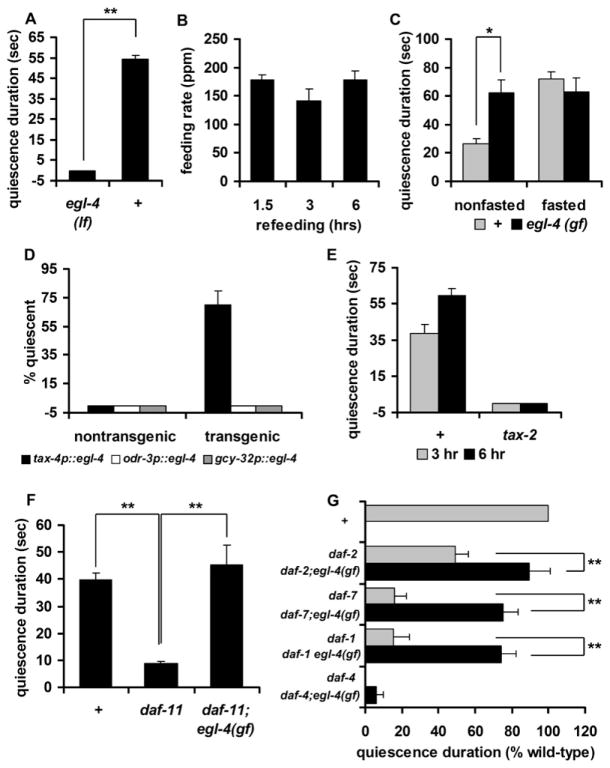
Peptide Signals Are Necessary for Quiescence
Many peptide signals such as NPY and CCK control feeding in mammals (Schwartz et al., 2000). Because quiescence mimics many aspects of satiety in mammals, we asked whether quiescence is also regulated by peptide signals. Mutations in egl-3 and egl-21, which encode proprotein convertase and carboxy-peptidase, respectively, eliminate most peptidergic signals (Husson et al., 2006; Jacob and Kaplan, 2003; Kass et al., 2001). We found that mutations in either gene abolished quiescence behavior (Figures 5A and 5B). unc-31 encodes calcium-dependent activator protein for secretion (CAPS) and functions in peptide secretion in the synapses (Ann et al., 1997). unc-31 mutants also failed to show quiescence (Figure 5C). On the other hand, mutants defective for nonpeptidergic neurotransmission via acetylcholine, serotonin, and dopamine showed little difference in quiescence behavior from wild-type worms (Table S2). This suggests that quiescence after fasting and refeeding is probably mediated by peptides rather than by nonpeptidergic neurotransmitters.
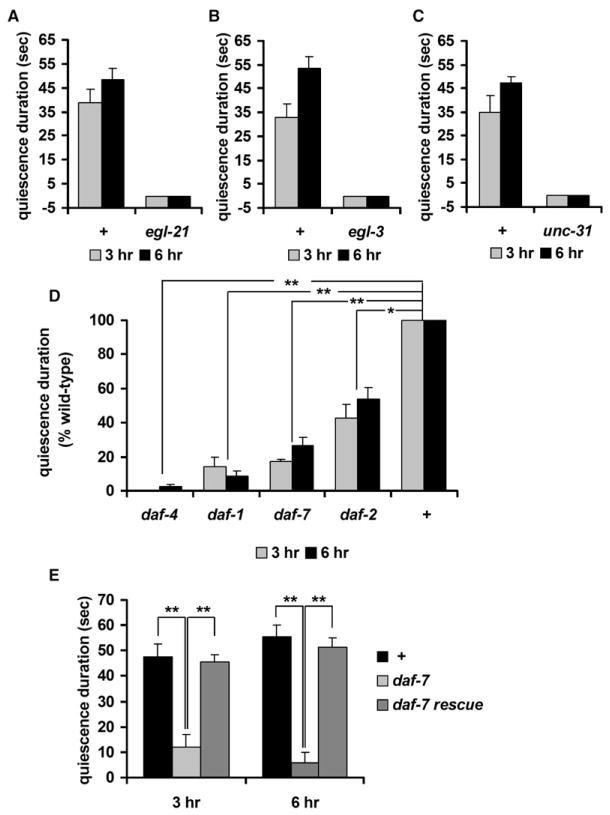
Figure 5. Peptide Signals Including Insulin and TGF-β Mediate Quiescence after Fasting and Refeeding.
In (A), (B), and (C), the x axis crosses the y axis at y = −5 so that the data for some mutants are visible.
(A) Quiescence duration of egl-21 mutants. egl-21 mutants failed to show quiescence after 3 (gray bars) or 6 (black bars) hr of refeeding.
(B) Quiescence duration of egl-3 mutants. egl-3 mutants failed to show quiescence after 3 (gray bars) or 6 (black bars) hr of refeeding. Four different alleles of egl-3 (nu349, gk238, n729, and nr2090) were tested, and all failed to show quiescence. The data from nr2090 are shown.
(C) Quiescence duration of unc-31 mutants. unc-31 mutants failed to show quiescence after 3 (gray bars) or 6 (black bars) hr of refeeding.
(D) Quiescence durations (expressed as a percent of wild-type) of daf-2 and TGF-β signaling mutants after 3 (gray bars) or 6 (black bars) hr of refeeding (see Experimental Procedures). (E) Expression of daf-7 in ASI neurons rescues the daf-7 quiescence defect.
Values are the mean ± SEM. *p < 0.005, **p < 0.001. + indicates wild-type.
Insulin and TGF-β Signaling Regulate Quiescence
Insulin is a nutrition signal and contributes to controlling food intake in mammals (Anika et al., 1980; Woods and Porte, 1983). Based on the high conservation of insulin signaling between mammals and worms (Kimura et al., 1997), we hypothesized that worm insulin signaling might regulate quiescence as well. An insulin receptor mutant, daf-2, showed shorter quiescence duration than wild-type (Figure 5D). This result supports the hypothesis that quiescence is a result of satiety because both behaviors are regulated by a common nutritional signal, insulin.
In worms, TGF-β signals favorable environmental characteristics such as presence of food (Patterson and Padgett, 2000). Expression of the C. elegans TGF-β DAF-7 is upregulated during refeeding after fasting (Wang and Kim, 2003), suggesting that DAF-7 mediates a food signal. We found that mutations in daf-7 attenuated quiescence after fasting and refeeding, as did mutations in daf-1 and daf-4, homologs of the TGF-β receptor type I and type II, respectively (Figure 5D). This suggests that DAF-7 may be necessary for quiescence after fasting and refeeding, probably as a food signal. In summary, we conclude that quiescence after fasting and refeeding is regulated by peptide signals such as insulin and TGF-β.
DAF-7 in ASI Neurons Regulates Quiescence
DAF-7 is expressed in a pair of ASI neurons (Ren et al., 1996). Unfavorable conditions such as shortage of food, high temperature, and high concentration of dauer pheromone induce dauer formation in worms. Worms become dauers even under favorable conditions if ASI neurons are killed (Bargmann and Horvitz, 1991). Recent findings suggest that ASI neurons are necessary for life-span extension by diet restriction (Bishop and Guarente, 2007). These results suggest a role of the ASI neurons in regulating or sensing food intake. When we replaced the wild-type DAF-7 gene product in the ASI neurons of daf-7 mutants using a gpa-4 promoter (Jansen et al., 1999), we completely rescued the quiescence defect (Figure 5E). Thus, quiescence after fasting and refeeding is mediated by daf-7 in ASI neurons, supporting the role of ASI neurons in regulating or sensing food intake.
PKG in TAX-2/4-Expressing Neurons Regulates Quiescence
It has been shown that the protein kinase G (PKG) EGL-4 is necessary in worms for olfactory adaptation, body size control, and determination of locomotor behavioral states (roaming versus dwelling) (Fujiwara et al., 2002; Hirose et al., 2003; L’Etoile et al., 2002). PKG in other invertebrates plays a role in food-seeking behavior (Ben-Shahar et al., 2002; Osborne et al., 1997). egl-4 loss-of-function mutants roam more and are larger than wild-type, while an egl-4(ad450) gain-of-function mutant is often found to be quiescent and is smaller than wild-type (Avery, 1993; Raizen et al., 2006). As described above, wild-type adults hardly show quiescence on DA837, whereas egl-4 gain-of-function mutants do. We reasoned that if egl-4 gain of function caused excessive quiescence, then egl-4 loss of function might abolish quiescence. In fact, egl-4 loss-of-function mutants did not show quiescence behavior at all, but constantly fed and moved, even after fasting and refeeding (Figures 6A and 6B). In contrast, nonfasted egl-4 gain-of-function mutants showed approximately double the quiescence duration of wild-type (Figure 6C). The quiescence duration of egl-4 gain-of-function mutants was not further enhanced by fasting, however, suggesting that the quiescence duration of nonfasted egl-4 gain-of-function mutants had already reached the maximum value (Figure 6C).
Figure 6. Insulin, cGMP, and TGF-β Signaling Regulate Quiescence through PKG in TAX-2/4-Expressing Neurons.
In (A), (D), and (E), the x axis crosses the y axis at y = −5 so that the data for some mutants are visible.
(A) Quiescence duration of egl-4 loss-of-function mutants (lf) after 3 hr of refeeding. Eleven egl-4(lf) mutants and thirteen wild-type (+) worms were used for the fasting/refeeding experiment.
(B) Feeding rates (measured in ppm) of egl-4(lf) mutants at the indicated time points during refeeding (n = 11).
(C) Quiescence duration of egl-4 gain-of-function mutants (gf) after 3 hr of isolation. For the nonfasted data, 19 nonfasted wild-type (+) and 20 nonfasted egl-4(gf) mutants were used. For the fasted data, 11 wild-type worms and 16 egl-4(gf) mutants were fasted and refed.
(D) Expression of egl-4 under the control of a tax-4 promoter (black bars) rescued the egl-4 quiescence behavior defect after 6 hr of refeeding, whereas expressing egl-4 under the control of either an odr-3 (white bars) or a gcy-32 (gray bars) promoter did not.
(E) Mutations in tax-2 abolish quiescence. tax-2 failed to show quiescence after 3 (gray bars) or 6 (black bars) hr of refeeding. Two different alleles of tax-2 (p671 and p694) were tested, and both failed to show quiescence. The data from p694 are shown.
(F) daf-11 is an upstream guanylyl cyclase (GCY) regulating EGL-4 in quiescence. Quiescence durations of daf-11 and daf-11; egl-4(gf) double mutants.
(G) Quiescence durations of TGF-β signaling mutants (gray bars) and double mutants with egl-4(gf) (black bars) calculated as percent of wild-type (see Experimental Procedures for SEM estimation).
Values are the mean ± SEM. *p < 0.005, **p < 0.001.
To determine the site of action of egl-4, we expressed wild-type egl-4 under the control of a tax-4 promoter and asked whether this expression rescued the quiescence defect. tax-4 encodes a cGMP-gated channel expressed in a dozen neurons (including ASI) that sense the worm’s environment and internal state (Komatsu et al., 1996). These neurons have critical roles in controlling body fat, determining body size, and changing feeding behaviors depending on environmental factors such as oxygen concentration (Coates and de Bono, 2002; Fujiwara et al., 2002; Mak et al., 2006). tax-4::egl-4 rescued quiescence in the egl-4 mutants (Figure 6D). In contrast, expressing egl-4 in a subset of TAX-4-expressing neurons using either an odr-3 promoter (to express in AWA, AWB, and AWC neurons) or a gcy-32 promoter (to express in AQR, PQR, and URX neurons) did not rescue quiescence (Figure 6D).
TAX-4 and TAX-2 are α and β subunits, respectively, of a cGMP-gated channel (Komatsu et al., 1996), and their neuronal expression overlaps. When we tested tax-2 mutants, they showed no quiescence (Figure 6E). (We were unable to test tax-4 mutants because, unlike tax-2 mutants, they often stray away from food.) These results suggest that the TAX-2/4-expressing neurons and their neuronal activities mediated by the TAX-2/4 cGMP-gated channel are necessary for quiescence after fasting and refeeding.
Insulin, cGMP, and TGF-β Signaling Regulate Quiescence through PKG
Because EGL-4 is a cGMP-dependent protein kinase, we searched for guanylyl cyclases (GCYs) that might activate it. We found that mutants of daf-11, which encodes a membrane-bound GCY (Birnby et al., 2000), showed a shorter quiescence duration than wild-type (Figure 6F). The egl-4 gain-of-function mutation suppressed the daf-11 defect in quiescence behavior, suggesting that daf-11 is upstream of egl-4, as expected if this signaling model were correct.
The egl-4 gain-of-function mutation also suppressed the daf-2, daf-1, and daf-7 defects, suggesting that daf-2, daf-1, and daf-7 are upstream of egl-4. Although the egl-4 gain-of-function mutation partially suppressed the daf-1 and daf-7 defects, it did not suppress that of daf-4 (Figure 6G). This suggests that TGF-β conveys satiety signals via egl-4-dependent and -independent pathways, consistent with other reports regarding the role of TGF-β pathways in dauer formation (Daniels et al., 2000; Kimura et al., 1997).
DISCUSSION
In this study, we report quiescence behavior in worms, which results in inhibition of food intake and movement. We propose that quiescence is a result of satiety because it depends on food quality, requires nutritional signals from the intestine, and depends on prior feeding history. Moreover, the behavioral sequence leading to quiescence in worms resembles the behavioral sequence of satiety in mammals. Quiescence after fasting and refeeding is regulated by insulin and neuropeptide signaling pathways, suggesting that it may share molecular mechanisms with the control of food intake in mammals. Based on our findings, we suggest a model wherein quiescence after fasting and refeeding induces signaling pathways in which PKG (EGL-4) is activated by insulin, cGMP, and TGF-β pathways (Figure 7).
Figure 7. Insulin, cGMP, and TGF-β Signaling Regulate Quiescence through PKG.
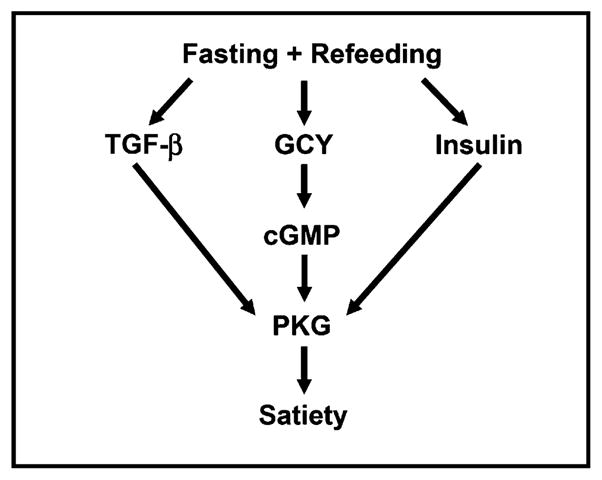
Quiescence after fasting and refeeding is controlled by a signaling pathway in which PKG (EGL-4) is activated by insulin, cGMP, and TGF-β pathways.
Quiescence is distinct from other, previously described C. elegans behavioral states. Worms alternate between two locomotor behavioral states, dwelling and roaming (Fujiwara et al., 2002). Dwelling and quiescence show superficial similarities: both attenuate locomotion, and EGL-4 is necessary in TAX-4 neurons for both. However, we found that the two behaviors were different in their details. Worms in the quiescent state are completely inactive in terms of both locomotion and feeding, unlike dwelling worms, which feed actively. In fact, during the initial refeeding period immediately after fasting, worms dwelled but fed actively. In addition, we did not find a correlation between the two behaviors in mutants that predominantly dwell (Table S3). This implies that, with the exception of EGL-4, the molecules that control the two behaviors are different. For these reasons, we conclude that quiescence and dwelling are distinct behaviors. Quiescence is also distinct from the enhanced slowing response (Ranganathan et al., 2000), because mod-1 mutants showed intact quiescence behavior (Table S2).
In worms, insulin, cGMP, and TGF-β pathways are involved in sensing a favorable environment and making the decision to keep growing and reproducing instead of becoming a dauer, a nonreproductive form specialized for long-term survival (Riddle et al., 1981). However, we do not believe that quiescence is simply related to dauer formation, for two reasons. First, we used temperature-sensitive mutants and maintained the worms so that they did not become dauers. Whatever function these dauer genes have in quiescence, it is at least not at the dauer-specific stage. Second, and more importantly, there is no clear correlation in strength between dauer formation and quiescence defects. For example, tax-2 and egl-4 are weak dauer-constitutive mutants (daf-c) but show the strongest defect in quiescence. All the other dauer-constitutive mutants (including daf-11, the strongest daf-c) still show quiescence, albeit briefly. Therefore, we suggest that these pathways that mediate the regulation of dauer formation by food may also regulate other food-dependent behaviors such as satiety, but through somewhat different mechanisms.
Interestingly, most mutants defective in quiescence behavior have darker intestines than wild-type. A dark intestine usually correlates with increased fat storage (McKay et al., 2003). In fact, some of these mutants, including daf-2 and daf-7, are known to store more fat than wild-type (Kimura et al., 1997). Our hypothesis that quiescence results from satiety strongly suggests that worms do not feed constantly but rather regulate their feeding depending on nutritional status and environment. Quiescence-defective mutants that have reduced “good conditions” signaling may be defective in regulating feeding, which might cause increased feeding and fat accumulation. Recent studies suggest that natriuretic peptide receptors, homologs of DAF-11, have a role in storing and degrading fat through PKG in adipose tissue (Sengenes et al., 2000) and that TGF-β signaling in neurons regulates adiposity during exercise (Ishikawa et al., 2006). Our results suggest the possibility of conserved linkage of these signaling pathways to regulation of feeding and metabolism in worms and mammals.
In conclusion, we have found that C. elegans has a conserved feeding behavior that shares characteristics typical of satiety behavior in mammals, making it a useful genetic model system to understand the regulation of appetite.
EXPERIMENTAL PROCEDURES
General Methods and Strains
Worms were cultured and handled as described previously (Sulston and Hodgkin, 1988) with the following modifications: worms were routinely grown on NGMSR plates (Avery, 1993). All worms were maintained at 20°C on E. coli strain HB101 unless indicated otherwise. The wild-type strain was C. elegans variant Bristol, strain N2. Mutant strains used were DA465 eat-2(ad465) II, IN2043 act-5(dt2017sd) III / eT1(III); + / eT1(V), CB1370 daf-2(e1370) III, DR1942 daf-2(e979ts) III, DR1942 daf-2(e979ts) III, FK234 egl-4(ks62) IV, UP1411 egl-4(ad450sd) IV; daf-11(sa195ts) V, DA521 egl-4(ad450sd) IV, DA2143 egl-4(ks62) IV; adEx2143[tax-4p::egl-4 rol-6p::GFP], DA2149 egl-4(ks62) IV; adEx2149[odr-3p::egl-4 rol-6p::GFP], DA2185 egl-4(ks62) IV; adEx2185[gcy-32p::egl-4 rol-6p::GFP], CB1372 daf-7(e1372ts) III, JT195 daf-11(sa195ts) V, DR40 daf-1(m40ts) IV, CB1364 daf-4(e1364ts) III, UP1395 daf-2(e1370) III; egl-4(ad450sd) IV, UP1438 daf-1(m40ts) egl-4(ad450sd) IV, UP1341 daf-7(e1372ts) III; egl-4(ad450sd) IV, UP1401 daf-4(e1364ts) III; egl-4(ad450sd) IV, PR671 tax-2(p671) I, PR694 tax-2(p694) I, CB1112 cat-2(e1112) II, LX703 dop-3(vs106) X, LX734 dop-2(vs105) V; dop-1(vs100) dop-3(vs106) X, BR1671 gar-2(by124) III, JD250 gar-3(lg1201) V, DA2100 ser-7(tm1325) X, DA2109 ser-7(tm1325) ser-1(ok345) X, DA1814 ser-1(ok345) X, GR1321 tph-1(mg280) II, MT9668 mod-1(ok103) V, FX903 dat-1(tm903) III, KP2018 egl-21(n476) IV, DA509 unc-31(e928) IV, KP1873 egl-3(nu349) V, VC461 egl-3(gk238) V, GR1328 egl-3(nr2090) V, MT1541 egl-3(n729) V, DA2202 daf-7(e1372ts) III; adEx2202[gpa-4p::daf-7 rol-6p::GFP], and DA2203 daf-7(e1372ts) III; adEx2203[gpa-4p::daf-7 rol-6p::GFP].
The standard “high-quality” and “low-quality” bacterial strains used were E. coli HB101 and E. coli DA837, respectively (Shtonda and Avery, 2006). It is unlikely that the nutritional content of these bacteria differs significantly—we believe rather that the significant difference is in the worm’s ability to ingest them. On NGMSR, DA837 tend to clump together, while HB101 do not, and a computer model suggests that these larger clumps will not be transported as efficiently within the pharynx (Shtonda and Avery, 2006). Unfortunately, we have no quantitative information on the net effect of this transport problem on nutrient uptake. The question cannot be answered simply by measuring food uptake, because it is likely that worms do not always absorb all of the nutrient that reaches the intestine (i.e., they release some by defecation) and that the efficiency of nutrient absorption may depend on the nutritional state of the worm. Thus, while we are confident that the worms obtain less nutritional benefit from DA837 than from HB101, we cannot quantify the ratio.
Quiescence Assay in Different Qualities of Food and for Malnourished Mutants
All worms were grown on the indicated food until young adulthood (approximately 9–12 hr after the L4 stage). To observe pumping and movement of worms precisely, 3 hr prior to observation we placed individual worms on plates seeded with the same food on which they had been grown. If a worm did not move and feed for more than 10 s (as shown in Movie S1), we considered it quiescent.
The Fasting/Refeeding Experiment
Worm Preparation
About 50 L4 larvae were transferred to HB101-seeded plates. Twelve hours later, when they had reached young adulthood, we transferred each worm with a platinum pick to an individual 60 mm NGMSR plate (Avery, 1993) without food. After 12 hr of fasting, we again transferred them singly to NGMSR plates seeded with HB101 for refeeding. Each worm was observed through a dissecting microscope (with 50× magnification) after refeeding for the indicated number of hours. For the nonfasted control, about 50 L4 larvae were transferred to HB101-seeded plates (same as the fasted samples). Twelve hours later, when they had reached young adulthood, these worms were transferred singly to NGMSR plates seeded with HB101 for the indicated periods of time.
We attempted to track individual worms during refeeding but did not succeed. The main problem was that at microscope resolution and magnification high enough to see pumping clearly, worms were disturbed by unknown factors and showed only inconsistent and brief quiescence.
Measuring Pumping Rate
Immediately after the worm was located at the indicated refeeding time point, the number of pumps was counted over the first 30 s. This number was then doubled to get “pumps per minute.”
Measuring Food Intake with GFP-Expressing E. coli HB101
Fasted and nonfasted worms were prepared as described above. GFP-expressing HB101 (a kind gift from D. Omura and R. Horvitz, Massachusetts Institute of Technology) was cultured overnight in LB broth and washed and resuspended in 50 μl of M9 buffer. Five microliters of this suspension was placed on each plate and allowed to dry. Fasted or nonfasted worms were placed on the bacterial lawn. Five minutes after transfer, worms were observed with a Zeiss Axioplan 2 imaging microscope, and pictures were taken with an AxioCam HRm camera and Openlab software (Improvision). Fluorescence intensity was measured and analyzed using ImageJ (http://rsb.info.nih.gov/ij/). We believe that these measurements are monotonic with total fluorescence, but they are unlikely to be linear.
Measuring Food Intake during Refeeding Using GFP-Expressing E. coli HB101
Fasted worms were prepared as described above. Worms were refed on non-GFP-expressing HB101 for the indicated length of time (0, 30, or 60 min) and then transferred to GFP-expressing HB101 for 5 min to measure food intake. Worms were observed, pictures were taken, and fluorescence intensity was analyzed as described above.
Measuring Food Intake Using BODIPY Dye
BODIPY dye (Molecular Probes, catalog number D-3822) was dissolved in DMSO at 1 mg/ml (a stock solution). The stock solution was diluted and spread on plates to achieve a nominal final concentration of 200 ng/ml based on the volume of agar on the plate. Fasted and nonfasted worms were placed on BODIPY plates seeded with HB101 for 15 min. Worms were observed, pictures were taken, and fluorescence intensity was analyzed as described above.
Measuring the Duration of Quiescence
The duration of quiescence was measured from the time when the worms were found to be quiescent until either continuous feeding or continuous locomotion occurred.
Quiescence Behavior Assays for Insulin Receptor, GCY, and TGF-β Signaling Mutants
Mutants and wild-type controls were grown at a permissive temperature (15°C) until they became L4 larvae, to prevent the mutants from becoming dauers. They were then transferred to and kept at a nonpermissive temperature (23°C) throughout the test period. The worms were prepared and observed as described for the fasting/refeeding experiment. All of the experiments were performed blindly with concurrent wild-type controls, and each was repeated independently three times. To combine the results into one graph, the ratio of the quiescence duration of mutants to that of concurrent wild-type controls was calculated from each experiment and then converted to a percent. Each value given is the mean ± SEM of the percents from three independent experiments.
Quiescence behavior assays for other mutants were performed as for insulin and TGF-β mutants, except that worms were grown and maintained at 20°C.
Nose Touch Test
Two groups (fasted and nonfasted) were prepared and observed as described for the fasting/refeeding experiment. After 3 hr of refeeding, each worm was checked to determine whether it was quiescent. If so, the tip of its nose was touched with an eyelash to disturb it (Kaplan and Horvitz, 1993). Once movement had been confirmed, observation continued to determine whether the animal returned to quiescence within 30 s. If it did not, it was scored as 0; if it did, it was scored as 1. The mean of the scores was calculated to get the probability to return to quiescence.
Satiety Behavior Assay for Transgenic Lines Expressing egl-4 in TAX-4-Expressing Neurons
The tax-4p::egl-4 and odr-3p::egl-4 DNA constructs were kind gifts of M. Fujiwara (Kyushu University Graduate School) and N. L’Étoile (University of California, Davis), respectively. The gcy-32p::egl-4 plasmid was constructed as follows: two primers were used to PCR amplify 902 bp of the gcy-32 promoter (Yu et al., 1997): YJ213f (gcy-32 promoter forward containing a SphI site), 5′-CCAAAATTGCATGCCCACTGATGATGTGATGAAGC-3′; YJ214r (gcy-32 promoter reverse containing a BamHI site), 5′-ATAGGATCCATTCATTATATT TTCCTTTCCGCTTTC-3′.
The plasmid containing tax-4p::egl-4 was digested with SphI and BamHI to remove the tax-4 promoter. This digested plasmid was ligated to the PCR product to replace the tax-4 promoter with the gcy-32 promoter. The promoter exchange was confirmed by sequencing.
GFP under the control of a rol-6 promoter was used as a transgenic marker. Stable transgenic lines were recognized by expression of green fluorescence in the second-generation progeny of worms microinjected with the DNA. Fifteen transgenic and fifteen nontransgenic siblings were prepared and observed as described for the fasting/refeeding experiment, and their quiescence behaviors were scored blind to their genotype. The experiment was performed twice for each of two independent transgenic lines.
DNA Construct and Injection for Expression of daf-7 in ASI Neurons
2.4 kb of the gpa-4 promoter region containing the 5′ end of daf-7 was generated by PCR using primers forward 5′-GACAGAAGACAGAGACTCGAG-3′, reverse 5′-TGCCATGAACATTGTTGAAAAGTGTTCACAAAATG-3′ (product 1). The daf-7 coding region containing the 3′ end of the gpa-4 promoter was generated by PCR using primers forward 5′-CACTTTTCAACAATGTTCATGGCAT CTTCACTCC-3′, reverse 5′-GACCTGACACCAAGTGTATGG-3′ (product 2). PCR products 1 and 2 were fused by PCR using primers forward 5′-GCGACTT TCGATACGTAGGTC-3′, reverse 5′-GTTACCGTTCAAGCAATTTCTCAG-3′ to produce 4.4 kb of the gpa-4 promoter fused to the daf-7 coding region. GFP under the control of a rol-6 promoter was used as a transgenic marker. Stable transgenic lines were recognized by expression of green fluorescence in the second-generation progeny of worms microinjected with the DNA. Ten transgenic and ten nontransgenic siblings were prepared and observed as described for the fasting/refeeding experiment, and their quiescence behaviors were scored blind to their genotype. The experiment was performed three times for each of two independent transgenic lines.
Statistics
The heteroscedastic two-tailed Student’s t test was used to assess the statistical significance ofdifferencesbetweentwo groups. All data are presented asmeans, and error bars represent SEM. p values are indicated in the figure legends.
Since the data in Figure 6G come from a single experiment, we estimated the standard error from the variation between worms in that experiment using the following equation:
where
q̄+ is the mean quiescence duration for wild-type
q̄m is the mean quiescence duration for mutant
r = q̄m/q̄+ is the ratio of mutant quiescence duration for wild-type
SE+ and SEm are the standard errors of q̄+ and q̄m
SEr is the estimated standard error of r
Supplementary Material
Acknowledgments
We thank M. Fujiwara, N. L’Étoile, and D. Riddle for DNA constructs; D. Omura and R. Horvitz for GFP-expressing E. coli; J. Waddle and J. Kaplan for C. elegans strains; S. Cameron, J. Maines, and K. Orth for reading the manuscript and providing comments; and O. Yilmaz, M. Cobb, B.M. Song, C.H. Kang, J. McKay, Z. Wang, and B. Shtonda for technical assistance and discussions. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. This study was supported by NIH grants DK074065 (Y.-j.Y. and L.A.) and NS048914 (D.M.R.) and Inha University and Korea Research Foundation grant KRF-2005-070-C00118 (J.K.).
Footnotes
Supplemental Data include three tables, one figure, and two movies and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/7/3/249/DC1/.
References
- Anika SM, Houpt TR, Houpt KA. Insulin as a satiety hormone. Physiol Behav. 1980;25:21–23. doi: 10.1016/0031-9384(80)90175-4. [DOI] [PubMed] [Google Scholar]
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Coates JC, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- Daniels SA, Ailion M, Thomas JH, Sengupta P. egl-4 acts through a transforming growth factor-beta/SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics. 2000;156:123–141. doi: 10.1093/genetics/156.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245:323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61:159–168. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C elegans. Development. 2003;130:1089–1099. doi: 10.1242/dev.00330. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Clynen E, Baggerman G, Janssen T, Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Mizunoya W, Shibakusa T, Inoue K, Fushiki T. Transforming growth factor-beta in the brain regulates fat metabolism during endurance exercise. Am J Physiol Endocrinol Metab. 2006;291:E1151–E1159. doi: 10.1152/ajpendo.00039.2006. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23:2122–2130. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- L’Etoile ND, Coburn CM, Eastham J, Kistler A, Gallegos G, Bargmann CI. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–1089. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- MacQueen AJ, Baggett JJ, Perumov N, Bauer RA, Januszewski T, Schriefer L, Waddle JA. ACT-5 is an essential Caenorhabditis el-egans actin required for intestinal microvilli formation. Mol Biol Cell. 2005;16:3247–3259. doi: 10.1091/mbc.E04-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G. Polygenic control of Caenorhabditis elegans fat storage. Nat Genet. 2006;38:363–368. doi: 10.1038/ng1739. [DOI] [PubMed] [Google Scholar]
- McKay RM, McKay JP, Avery L, Graff JM. C elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003;4:131–142. doi: 10.1016/s1534-5807(02)00411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Patterson GI, Padgett RW. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Cullison KM, Pack AI, Sundaram MV. A novel gain-of-function mutant of the cyclic GMP-dependent protein kinase egl-4 affects multiple physiological processes in Caenorhabditis elegans. Genetics. 2006;173:177–187. doi: 10.1534/genetics.106.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–475. doi: 10.1038/35044083. [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Hodgkin JG. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY, USA: Cold Spring Harbor Press; 1988. pp. 587–606. [Google Scholar]
- Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr The role of insulin as a satiety factor in the central nervous system. Adv Metab Disord. 1983;10:457–468. doi: 10.1016/b978-0-12-027310-2.50024-4. [DOI] [PubMed] [Google Scholar]
- You YJ, Kim J, Cobb M, Avery L. Starvation activates MAP kinase through the muscarinic acetylcholine pathway in Caenorhabditis elegans pharynx. Cell Metab. 2006;3:237–245. doi: 10.1016/j.cmet.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.