Figure 7. IRF7 Activation Requires the Formation of an Interferon Signaling Compartment.
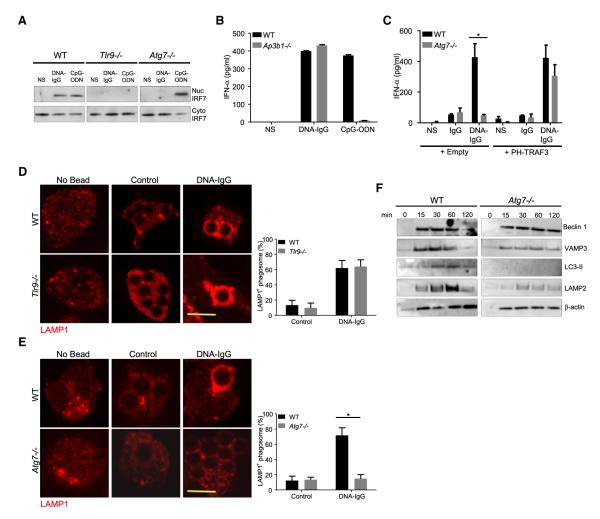
(A) Fetal liver-derived pDCs from WT, Tlr9−/−, or Atg7−/− mice were left untreated (NS) or were treated with a combination of CG50 plasmid DNA and DNA antibody E11 (DNA-IgG) or with 5 μM ODN 1585 (CpG-ODN) for 6 hr. The presence of IRF7 in nuclear and cytoplasmic extracts was assessed by immunoblotting. Data presented are representative of three independent experiments.
(B) Bone marrow-derived pDCs from WT and Ap3b1−/− mice were incubated for 24 hr with DNA-IgGs or ODN 1585 (CpG-ODN) as in (A). IFN-α in supernatant was measured by ELISA. Representative data from at least two independent experiments are presented. Data are presented as mean ± SD.
(C) Fetal liver-derived pDCs from WT and Atg7−/− mice transiently expressing PH-TRAF3 or an empty vector were incubated for 24 hr with DNA-IgG as in (A). No treatment (−) or DNA antibody E11 alone (IgG) were used as control. IFN-α in supernatant was measured by ELISA. Representative data from at least two independent experiments are presented. Data are presented as mean ± SD.
(D and E) Fetal liver-derived pDCs were fed with uncoated beads (Control) or DNA-IgG-coated beads for 4 hr. Cells were then fixed and LAMP1 was detected by immunofluorescence. Confocal images for TLR9−/− cells (D) and Atg7−/− (E) pDCs were obtained and representative images shown. The percentage of phagosomes that were positive for LAMP1 upon bead ingestion was quantified for three independent experiments (n ≥ 50 phagosomes per group). Data are presented as mean ± SD (*p value < 0.05). Scale bars represent 5 μm.
(F) Immunoblotting analysis of phagosome proteins. Fetal liver-derived pDCs from WT and Atg7−/− mice were allowed to phagocytose latex beads coated with DNA-IgG for the indicated time. Phagosomes were purified using sucrose gradient as described in experimental procedures. Phagosome proteins were solubilized in SDS-PAGE and blotted with the indicated antibodies. The results presented are representative of three independent experiments.