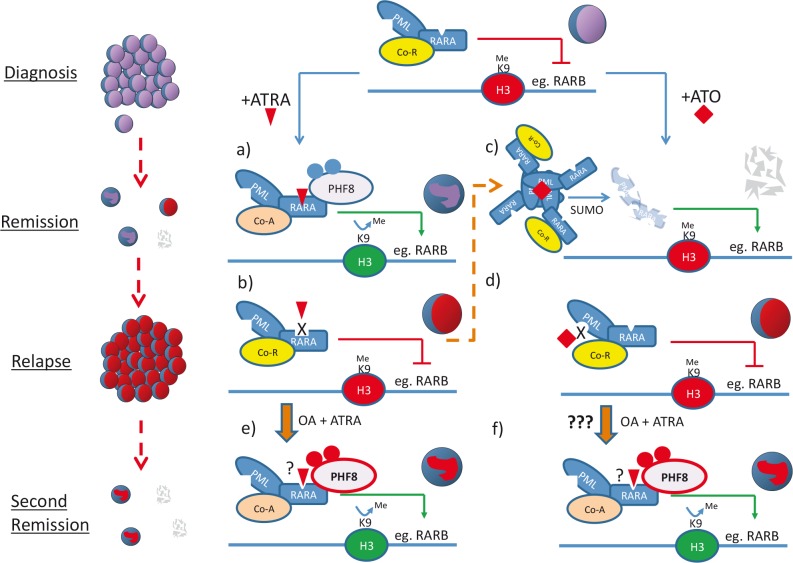
Figure 1. Schematic diagram illustrates the molecular basis of ATRA/ATO treatments and the potential avenues of overcoming treatment resistance by modulating PHF8 activity in APL.
PML-RARA aberrantly recruits co-repressor complexes (Co-R, yellow oval) and epigenetically suppresses expression of downstream target genes critical for differentiation and tumor suppression.
(a) ATRA (red triangle) binds to the LBD of RARA moiety and facilitates the exchange of co-repressor with co-activator complexes (co-A, pink oval). ATRA also induces phosphorylation of PHF8 that is recruited by the RARA fusion to remove suppressive H3K9me epigenetic mark and subsequently converts repressive chromatin (red H3 oval) to permissive chromatin (green H3 oval) for active transcriptional programs leading to cell differentiation.
(b) However, long-term exposure to ATRA may cause selection or induction of leukemic clones carrying mutations on the LBD of the RARA moiety or aberrant transcription repression complexes that could not be dissociated by pharmacological level of ATRA treatment. Hence cells become ATRA-resistant and APL relapses.
(c) On the other hand, ATO (red diamond) that leads to degradation of PML-RARA has been shown effective in inducing complete remissions even for ATRA-resistant APL (thin dashed orange arrow). In contrast to ATRA, a high dose of ATO results in apoptosis of APL cells.
(d) Again, long-term treatment of ATO can select/induce resistant leukemic clone(s) carrying mutations on the PML moiety affecting ATO-mediated degradation. ATO-resistant clone expands and disease relapses.
(e) and (f) Over-expression or hyperphosphorylation of PHF8 (red), which could be achieved by phosphatase inhibitor OA treatment, re-sensitizes ATRA-resistant APL to ATRA, probably through epigenetic activation of downstream target gene expression (e). Since ATO-resistance in APL is largely due to the mutation on PML moiety, PHF8 can still in principle be effective in targeting the RARA moiety of the fusion proteins and induces cell differentiation (f).