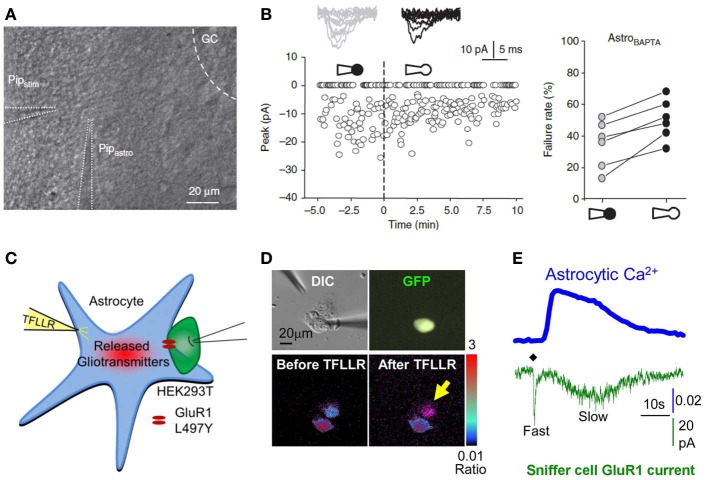
Figure 5.
Interfering with astrocytes influences neuronal activity. (A,B) Infusion of BAPTA in the astrocytic network decreases synaptic efficacy in minimal stimulation conditions. (A) Experimental arrangement showing whole cell patch clamp of an astrocyte (PipAstro) present in the dendritic tree of the recorded granule cell and 20–30 μm away from the stimulating pipette (Pipstim). The intra-pipette solution for patch clamp contains the Ca2+ chelator BAPTA. (B) Failure rate changes in granule cells after breaking the cell membrane and dialyzing the astrocyte with the intra-pipette solution containing BAPTA. (C–E) Measure of astrocytic glutamate release using the sniffer patch technique. (C) Schematic illustration of the sniffer-patch. The left pipette (yellow) is used for pressure application of the PAR-1 agonist TFLLR. This stimulation results in glutamate release (red cloud) producing a measureable inward current in the HEK293T sensor cell (green) expressing GluR1-L497Y containing AMPA receptors (red). (D) Images for sniffer patch. DIC image (upper left): two cells with two glass pipettes. GFP image (upper right): sensor cell expressing GluR1-L497Y and GFP. Pseudocolor images: Fura-2 loaded astrocyte (source cell) and sensor cells before (lower left) and after stimulation (lower right). Yellow arrow: increased Ca2+ in astrocyte. (E) Representative traces recorded from the sniffer-patch technique. Blue trace: Ca2+ transient recorded from astrocyte. Green trace: whole cell current recorded from sensor cell (voltage clamped at −70 mV) upon TFLLR pressure application. Diamond: TFLLR application (10 psi, 100 ms, 500 μ M). Adapted with permission from Di Castro et al. (2011) (A,B) and Woo et al. (2012) (C–E).