Abstract
Background
We considered that women with prior preterm birth (PTB) would have evidence of subclinical atherosclerosis, endothelial dysfunction, and arterial stiffness.
Methods
Four to 12 years after pregnancy, blood pressure and fasting lipids were analyzed, and women underwent evaluation, following standardized protocols, of carotid intima-media thickness (IMT), brachial flow-mediated dilation (FMD), and pulse wave velocity (PWV). Women with prior preterm (<37 weeks, n=181) or term births (>= 37 weeks, n=306) were compared. Those with preeclampsia or term small-for-gestational-age (SGA) births were excluded.
Results
Women with a prior preterm vs. term birth had higher blood pressure, on average, and a more atherogenic lipid profile. They also had marginally higher IMT (0.579 standard error [SE] 0.005—vs. 0.567 [0.004] mm, p=0.06), adjusted for body size, demographics, and smoking. IMT differences were greater among those with non-preeclamptic-indicated PTB (0.034 mm, p=0.05) and PTB<34 weeks (0.024 mm, p=0.04) compared to those with term births. These differences appeared to be explained in part by the atherogenic lipid elevations in women with preterm birth. Women with prior PTB<34 weeks tended to have lower FMD, but results were not statistically significant. PWV did not differ according to PTB.
Conclusions
In the decade following pregnancy, women with non-preeclamptic-indicated PTB or PTB delivered before 34 weeks had higher blood pressure, atherogenic lipids, and IMT compared to women with term births. There may be subgroups of women with a prior PTB with excess cardiovascular risk that is detectable before overt clinical disease.
Introduction
Preterm birth (PTB) affects more than 12% of births in the United States. Women with a history of PTB have a two- to threefold increased risk of cardiovascular disease (CVD),1–5 but mechanisms relating these conditions are not understood. Associations between preeclampsia and maternal cardiovascular risk factors are well established; however, some1,4 but not all studies6 indicate that non-preeclamptic preterm births are related to maternal cardiovascular risk.
Women with PTB have elevated cholesterol before and during pregnancy.7–10 We have previously reported that they have dyslipidemia, higher blood pressure, and evidence of metabolic syndrome—a cluster of cardiovascular risk factors—in the decade after pregnancies delivered preterm without preeclamspsia.11 In the current study, we hypothesized that this atherogenic profile in women with a prior non-preeclamptic preterm birth would be related to subclinical atherosclerosis, endothelial dysfunction, and arterial stiffness.
Atherosclerotic vascular disease is a chronic inflammatory, fibroproliferative disease of large and medium-sized arteries fueled by lipid.12 Artery wall thickening as assessed by intima-media thickness (IMT) is an early stage of arterial injury and atherosclerosis and is detectable and predictive of CVD events among young women.13–15 Endothelial dysfunction, an early and modulating process in the development of atherosclerosis, can be quantified by flow-mediated dilation (FMD), which represents the endothelium-dependent relaxation of the brachial artery owing to increased blood flow.16,17 Pulse wave velocity (PWV) is a measure of arterial stiffness that has been linked to insulin resistance, metabolic syndrome, and central adiposity, as well as risk of cardiovascular events.18–20 We considered that the metabolic syndrome detected in women with a prior PTB vs. term birth would be related to evidence of subclinical cardiovascular disease as assessed by IMT, FMD, and PWV. Given the heterogeneity of PTB, we also examined whether subgroups of women with preterm birth according to clinical presentation and gestational age would have different cardiovascular risk profiles in the decade after pregnancy.
Subjects and Methods
The Women and Infant Study of Healthy Hearts (WISH) is a cohort study of cardiovascular factors assessed among women 4 to 12 years after delivery of singleton infants who were preterm, small for gestational age (SGA), or delivered at term with normal growth (n=702). The University of Pittsburgh Institutional Review Board (IRB) approved all study procedures. Eligibility and recruitment results have been reported previously.11 Briefly, eligible women were those who gave birth between 1997 and 2002 at Magee-Womens Hospital in Pittsburgh and who did not have preeclampsia or prepregnancy hypertension or diabetes. A total of 702 women provided informed consent and were enrolled. Those who delivered SGA infants at term (n=190) were excluded from this analysis, as the pathways involved in term SGA and PTB are distinct, and therefore the postpregnancy consequences may also be different. There were six cases of SGA in the preterm group, and we replicated analyses after excluding them to ensure that results were not related to growth restriction. No results were affected, so these cases were retained in the current study. We also excluded women who reported their race or ethnicity as other than white or African American, owing to small numbers (n=12). We excluded women with gestational diabetes (n=11), given its well-established relation to later-life cardiovascular disease. Women with triglycerides>400 mg/dL (n=2) were also excluded, as the estimation of low-density lipoprotein cholesterol (LDL-C) is not valid in these individuals.21 The final study population was 487 women.
Delivery characteristics were abstracted from hospital birth records and included gestational age (based mainly on prenatal ultrasounds). Women were categorized as having delivered preterm (<37 weeks gestation, n=181) or term; the preterm group was further divided into those delivered <34 weeks (n=55) and 34–<36 weeks (n=126). Preterm births were also categorized as spontaneous (following spontaneous premature membrane rupture or preterm labor) or medically indicated, as the processes leading to these clinical presentations are likely different. By design, women with preeclampsia were excluded, and the medically indicated preterm births in our study were related to placenta previa or abruption, suspected growth restriction, and other fetal or maternal conditions. Cases of gestational hypertension (blood pressure above 140/90 without proteinuria, n=8) were included. Women with term, non-SGA infants (>10th percentile) were the referent for all analyses (n=306).
B-mode ultrasound images of the right and left distal common carotid artery, carotid bulb, and the first centimeter of the internal carotid artery were obtained in diastole. Semiautomated edge-detection software (Artery Measurement System, Gothenburg, Sweden) was used to identify the lumen-intima and media-adventitia interfaces and measure across 1 cm segments of the near and far walls of the common carotid artery and the far wall of the bulb and internal carotid artery. Mean IMT values across these eight sites were averaged to obtain mean average IMT. Reproducibility of IMT measures was excellent, with an intraclass correlation coefficient between sonographers of ≥0.87 and between readers of 0.92. Trained sonographers using a standardized protocol measured FMD after 10 minutes of supine rest by use of high-resolution B-mode ultrasound imaging of the right brachial artery, from 4 to 10 cm proximal to the antecubital crease. Images were obtained at rest (baseline) and after 4 minutes of forearm blood flow occlusion (postdeflation) with a pneumatic tourniquet set to 50 mm Hg above the participant's systolic blood pressure. For baseline diameters, digitized images were captured on the R wave for 20 seconds. Immediately after deflation, images were captured on the R wave for 3 minutes. Trained readers measured the arterial diameter as the distance between the proximal and distal arterial wall media-adventitia interfaces. All images for this study were read by one reader using the Brachial Analysis System (Medical Imaging Applications [MIA], University of Iowa) software developed at the University of Iowa.22 FMD was calculated as the maximum percentage of change in arterial diameter, relative to baseline. Reproducibility of FMD was fair, with an intraclass correlation coefficient between sonographers of ≥0.60 and within reader of 0.67. Carotid-femoral PWV was measured by taking simultaneous recordings of the pressure waveforms from the right common carotid and right femoral artery, using the automated Complior SP (Artech Medical, Pantin, France). PWV (cm/sec) was calculated as the distance traveled by the pressure wave between arterial sites of interest divided by the time delay (or transit time) between the respective waveforms. Three data-collection runs were performed and averaged, each obtaining a minimum of 10 pairs of simultaneously recorded pressure waveforms. The distance between the sampling sites (the carotid artery and the femoral artery) was measured over the surface of the body with a tape measure and calculated by the indirect method (carotid–femoral distance=((suprasternal–umbilicus)+(umbilicus–femoral))−(carotid–suprasternal)).23 Intraclass correlations of 0.75–0.91 for between-technician comparisons and 0.88–0.95 for within-technician comparisons were obtained.
Fasting blood samples were collected at the same study visit. All measurements were completed at the University of Pittsburgh's Nutrition Lab in the Department of Epidemiology, which is certified under Clinical Laboratory Improvement Amendments (CLIA) and participates in the CDC-NHLBI (National Heart, Lung, and Blood Institute) Lipid Standardization and College of American Pathologists' Proficiency Programs. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured under standard enzymatic procedures.24–26 LDL-C was evaluated using the Friedewald calculation.21 The coefficient of variation (CV) ranged from 1.3% to 6.5%. Apolipoprotein B (ApoB) was analyzed by using a variation of the Boehringer Mannheim turbidimetric procedure; the coefficient of variation was 9.8%.
Standard methods were used to evaluate blood pressure; a sphygmomanometer was used at the study visit to obtain the mean of three measurements following a 10-minute rest, and body mass index (BMI, kg/m2) was calculated from measured height and weight. Waist circumference was assessed in centimeters with a tape measure at the umbilicus. Women completed a structured interview to assess pregnancy and medical history, demographics, and lifestyle characteristics. Women reported the outcomes of all pregnancies before and following the index birth, including gestational age and birth weight. Smoking status and number of cigarettes smoked were assessed during pregnancy and at the postpartum study visit. Women also reported the first day of the last menstrual period; days from menses to the study visit were calculated because some markers may change during the menstrual cycle.27,28 Menopause was defined as having no menstrual periods during the previous 12 months, surgical removal of both ovaries, or age greater than 55 accompanied by use of estrogen, hormone therapy, or a hysterectomy. Weekly alcohol consumption was reported, and regular use was defined as consumption of some alcohol at least once a week. Physical activity was reported using the Paffenbarger Physical Activity Questionnaire29 and analyzed as total hours of physical activity expenditure per week (metabolic equivalent of task, or MET, hours/week).
Characteristics of women with preterm vs. term births were compared, using chi-square tests for categorical variables, t-tests for continuous variables, or analysis of variance (ANOVA) with post hoc pairwise comparisons for PTB subtypes using Dunnett's test. The Wilcoxon rank- sum test was used instead of the t-test if the normality assumption did not hold, based on quantile-quantile plots, histograms, and the Shapiro-Wilk test for normality. Women with term births were the referent for all analyses. IMT, FMD, and PWV were evaluated as continuous outcomes according to preterm birth status and related to gestational age in weeks, using linear regression. Results are also presented as adjusted means. All tests were two-sided, with statistical significance set at p-value=0.05.
To obtain noncollinear covariates for our regression model, we used variable clustering to create highly correlated groups of variables that describe the same feature.30 As described previously,11 we applied all covariates of interest (in Table 1) to a hierarchical clustering graph and found three significant variable clusters. The first included BMI and weight. The second cluster included education, income, race, age at index birth, and age at baseline. The third variable cluster included smoking during pregnancy, current smoking, and ever smoking, as well as amount smoked during pregnancy, number of years smoked, number of cigarettes smoked at baseline, and pack-years smoked among ever users. We then used principal- component analysis to create three new variables that were included in our final models as covariates. Additional adjustment for menstrual cycle phase did not affect estimates and was not included in the final models. The assumptions of linear regression models were checked, using residual plots and the White test for homoscedasticity.
Table 1.
Characteristics and Clinical Variables According to Pregnancy Outcome; Data Expressed as Mean (±Standard Deviation) or Number(%)
| Term n=306 | Preterm n=181 | pa | |
|---|---|---|---|
| Index pregnancy (1997–2002) | |||
| Age (years) | 28.5 (6.9) | 30.5 (6.8) | <0.01 |
| Prepregnancy weight (kg) | 66.3 (18.6) | 65.3 (13.2) | 0.68 |
| African American (%) | 103 (34) | 46 (26) | 0.06 |
| Gestational age (week) | 39.3 (1.1) | 34.0 (2.5) | <0.01 |
| Multiparous (%) | 269 (88) | 152 (84) | 0.34 |
| First born: index pregnancy (%) | 123 (40) | 70 (39) | 0.74 |
| Additional preterm births (before/after index) (%) | 38 (12) | 54 (30) | <0.01 |
| Gestational hypertension (%) | 6 (2) | 2 (1) | 0.47 |
| Smoking during pregnancy (%) | 59 (21) | 30 (19) | 0.61 |
| Study visit (2005–2009) | |||
| Time from index pregnancy to interview (years) | 8.0 (1.7) | 8.5 (1.7) | 0.06 |
| Age (years) | 36.5 (7.4) | 39.0 (7.0) | <0.01 |
| Tobacco use: ever (%) | 134 (44) | 97 (54) | 0.04 |
| Tobacco use: current (%) | 76 (25) | 60 (33) | 0.08 |
| Pack-years smokedb | 4.2 (5.9) | 4.6 (6.5) | 0.99 |
| Body mass index (kg/m2) | 27.8 (7.0) | 27.3 (6.6) | 0.44 |
| Waist circumference (cm) | 92.5 (15.8) | 91.7 (15.1) | 0.59 |
| Systolic blood pressure (mm Hg) | 106.7 (10.4) | 109.0 (12.3) | 0.05 |
| Diastolic blood pressure (mm Hg) | 69.8 (8.3) | 71.5 (7.8) | 0.03 |
| Statin use: current (%) | 3 (1) | 4 (2) | 0.27 |
| Oral contraceptive use (%) | 44 (14) | 18 (10) | 0.16 |
| Menopause (%) | 18 (6) | 22 (12) | 0.01 |
| Alcohol consumption: regularb (%) | 108 (35) | 61 (34) | 0.82 |
| Physical activity (MET hours/week) | 15.9 (32.8) | 12.1 (18.0) | 0.21 |
| Education (%) | 0.58 | ||
| <High school | 20 (7) | 7 (4) | |
| High school | 88 (29) | 49 (27) | |
| College | 159 (52) | 99 (55) | |
| >College | 39 (13) | 26 (14) | |
| Income (%) | 0.08 | ||
| Less than $20,000 | 80 (26) | 37 (20) | |
| $20,000–<$50,000 | 60 (20) | 50 (28) | |
| $50,000–<$100,000 | 93 (30) | 53 (29) | |
| >$100,000 | 62 (20) | 28 (15) | |
| Don't know | 11 (4) | 13 (7) | |
| Insurance (%) | <0.01 | ||
| Medicaid | 77 (25) | 19 (11) | |
| Medicare | 8 (3) | 5 (3) | |
| Private | 205 (67) | 146 (81) | |
| None | 16 (5) | 11 (6) | |
| Menstrual cycle phase at study visit (%) | 0.09 | ||
| <14 days of menses | 147 (48) | 76 (42) | |
| >= 14 days of menses | 159 (52) | 105 (58) | |
| Family history (%) | |||
| Hypertension | 175 (57) | 109 (60) | 0.51 |
| Diabetes | 19 (6) | 9 (5) | 0.57 |
| Heart disease | 73 (24) | 54 (30) | 0.15 |
| Stroke | 36 (12) | 24 (13) | 0.63 |
| Preeclampsia | 34 (11) | 18 (10) | 0.69 |
Student's t-test for means and χ2 test for categorical variables. The Wilcoxon rank-sum test was used to compare prepregnancy weight, pack-years smoked, and physical activity.
Pack years smoked among ever users (at least 200 cigarettes in lifetime). Regular alcohol use was defined as drinking beer, wine, or liquor at least once a week.
MET, metabolic equivalent of task.
Because atherogenic lipids and blood pressure may mediate the association between prior PTB and subclinical vascular measures, we did not include them as covariates in primary models.31 Instead, we compared a model that included these factors modeled as a lipid cluster (comprising total cholesterol, LDL-C, HDL-C, ApoB, and triglycerides) and systolic blood pressure (SBP), preterm birth, and covariates to a model in which the lipid cluster and SBP were omitted. We also tested for interaction between the lipid cluster and preterm birth, and SBP and preterm birth.
Analyses were performed with SAS (version 9.2, SAS Institute, Inc., Cary, NC) and R (version 2.6.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Women with a prior non-preeclamptic preterm birth tended to be older and have modestly higher rates of smoking after pregnancy and were less likely to have Medicaid health insurance compared to those with term births (Table 1). As expected, those women were more likely to report that they had additional preterm births.
Women with prior non-preeclamptic preterm vs. term births had higher mean systolic (109.1 vs. 106.7 mm Hg, p=0.03) and diastolic (71.5 vs. 69.8 mm Hg, p=0.03) blood pressures after adjusting for body size, socioeconomic status (SES) and smoking characteristics (Table 2). Women with spontaneous PTBs had blood pressure elevations that were intermediary between those measured in women with term and non-preeclamptic- indicated PTB. Women with a prior preterm birth also had higher total cholesterol (198.2 vs. 185.7 mg/dL, p<0.01), LDL-C (118.4 vs. 108.9 mg/dL, p<0.01), triglycerides (112.2 vs. 99.4 mg/dL, p=0.02), and ApoB concentrations (88.6 vs. 83.5, p=0.03) compared to women with term births. When evaluated according to clinical presentation and timing of delivery, women with all preterm birth subtypes in general had higher lipids compared to women with term births. Women with indicated PTBs, however, had higher HDL-C, and triglycerides were not different from those of women with term births.
Table 2.
Maternal Lipids (mg/dL and Blood Pressure (mm Hg) According to Prior Preterm Birth Status, Mean (Standard Deviation)
| |
All preterm birth |
Clinical presentation |
Gestational age at delivery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusteda | Term | Preterm | Spontaneous | Indicated | 34–<37 Weeks | <34 weeks | |||||
| Cholesterol | 185.7 (39.0) | 198.2 (36.2) | <0.01 | 196.9 (35.2) | <0.01 | 205.2 (41.4) | 0.01 | 198.1 (33.2) | <0.01 | 199.0 (42.7) | 0.02 |
| LDL cholesterol | 108.9 (33.2) | 118.4 (30.3) | <0.01 | 118.1 (29.9) | 0.01 | 120.5 (32.2) | 0.06 | 118.2 (28.9) | 0.01 | 119.1 (33.1) | 0.04 |
| Triglycerides | 99.4 (54.0) | 112.2 (66.9) | 0.02 | 114.0 (67.4) | 0.02 | 104.4 (64.8) | 0.54 | 109.6 (56.7) | 0.09 | 118.7 (85.8) | 0.02 |
| HDL cholesterol | 56.6 (14.2) | 57.4 (15.1) | 0.48 | 56.6 (14.5) | 0.77 | 63.8 (17.3) | <0.01 | 58.0 (15.3) | 0.30 | 56.2 (14.9) | 0.79 |
| Apolipoprotein-B | 83.5 (25.6) | 88.6 (22.9) | 0.03 | 88.6 (22.3) | 0.05 | 89.9 (25.4) | 0.24 | 86.6 (21.0) | 0.27 | 93.3 (25.9) | <0.01 |
| Systolic blood pressure | 106.7 (10.5) | 109.1 (12.3) | 0.03 | 108.6 (11.8) | 0.08 | 110.5 (14.6) | 0.05 | 109.1 (12.4) | 0.04 | 108.7 (12.2) | 0.21 |
| Diastolic blood pressure | 69.7 (8.3) | 71.6 (7.8) | 0.02 | 71.2 (7.2) | 0.08 | 73.3 (10.4) | 0.03 | 71.6 (0.05) | 0.04 | 71.7 (8.1) | 0.10 |
All measures are adjusted for body size cluster (BMI and waist circumference), SES cluster (mother's age at baseline, mother's age at index birth, race, education, and income), and smoking cluster (smoking during pregnancy (yes/no), smoked, number of years smoked (among ever smokers) amount smoked during pregnancy, current/not current smoking, ever smoking (yes/no), number of cigarettes).
BMI, body mass index; HDL high-density lipoprotein; LDL, low-density lipoprotein; SES, socioeconomic status.
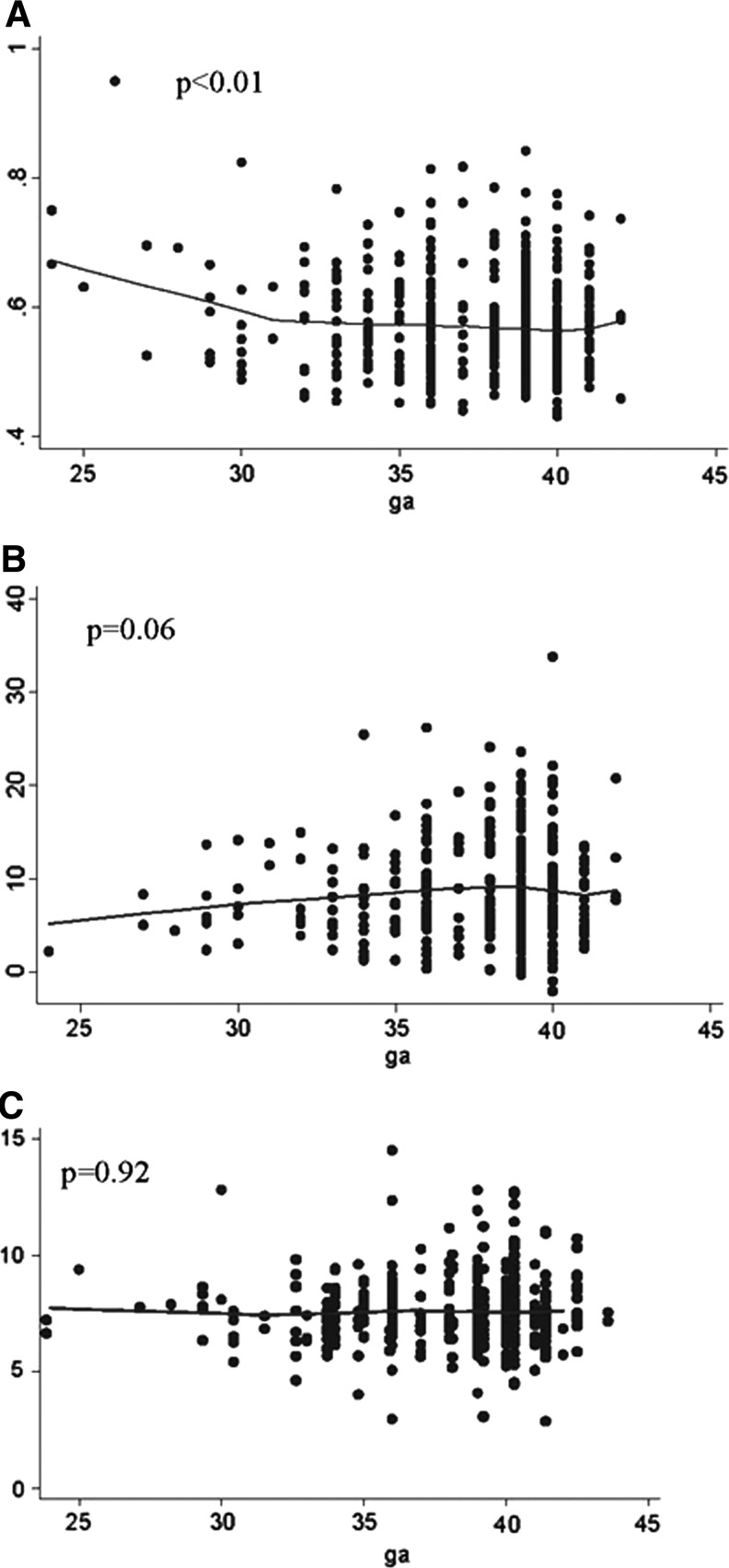
The mean IMT for women with preterm vs. term births was marginally higher after adjustment for covariates (difference 0.015, p=0.06; Table 3). Women with non-preeclamptic-indicated preterm births (but not spontaneous PTB) had higher mean IMTs compared to women with term births (difference 0.034 mm, p=0.05), as did those with preterm births delivered<34 weeks (difference 0.024, p=0.04). We tested for differences in brachial diameters between mothers delivered preterm vs. term and found no significant differences. When the gestational age of the index birth was evaluated continuously, IMT increased as the gestational age of the birth decreased (beta=–0.003, p<0.01), adjusted for covariates (Fig. 1, panel A). Removal of the one potential outlier did not change the trend of this association, and the results remained significant (p=0.03).
Table 3.
Subclinical Vascular Measures According to Prior Preterm Birth Status; Referent: Women with Term Births
| |
All preterm birth |
Clinical presentation |
Gestational age at delivery |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Preterm | p | Spontaneous | p | Indicated | p | 34–<37 weeks | p | <34 weeks | p |
| Intima-media thickness (mm) | 0.015 (0.007) | 0.06 | 0.118 (0.008) | 0.14 | 0.034 (0.014) | 0.05 | 0.011 (0.008) | 0.27 | 0.024 (0.011) | 0.04 |
| Flow-mediated dilation (%)c | −0.960 (0.665) | 0.11 | −1.038 (0.712) | 0.11 | –0.702 (1.427) | 0.55 | −0.636 (0.792) | 0.34 | −1.666 (1.023) | 0.07 |
| Pulse Wave Velocity (m/s) | −0.009 (0.154) | 0.94 | 0.001 (0.162) | 0.97 | –0.077 (0.324) | 0.81 | 0.001 (0.162) | 0.56 | −0.270 (0.241) | 0.26 |
| Model 2b | Preterm | p | Spontaneous | p | Indicated | p | 34–<37 weeks | p | <34 weeks | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Intima-media thickness (mm) | 0.007 (0.007) | 0.28 | 0.006 (0.006) | 0.31 | 0.020 (0.014) | 0.15 | 0.003 (0.008) | 0.71 | 0.016 (0.011) | 0.14 |
| Flow-mediated dilation (%) | −0.755 (0.684) | 0.27 | −0.912 (0.730) | 0.21 | −0.205 (0.864) | 0.89 | −0.571 (0.809) | 0.48 | −1.316 (1.05) | 0.21 |
| Pulse wave velocity (m/s) | −0.088 (0.146) | 0.54 | −0.080 (0.154) | 0.61 | −0.148 (0.316) | 0.64 | −0.080 (0.154) | 0.89 | −0.363 (0.233) | 0.12 |
Model 1: All measures are adjusted for body size cluster (BMI and waist circumference), SES cluster (mother's age at cardiovascular study visit, mother's age at index birth, race, education, and income), and smoking cluster (smoking during pregnancy (yes/no), smoked, number of years smoked (among ever smokers) amount smoked during pregnancy, current/not current smoking, ever smoking (yes/no), number of cigarettes).
Model 2: All measures are adjusted for factors in Model 1, with additional adjustment for lipid cluster (cholesterol, LDL-C, HDL-C, ApoB, and triglycerides), and systolic blood pressure.
Flow-mediated dilation is also adjusted for baseline diameter.
ApoB, apolipoprotein-B, HDL-C, high-density liproprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
FIG. 1.
Vascular measures related to gestational age of index pregnancy. (A) Intima-media thickness; (B) Flow-mediated dilation; (C) Pulse wave velocity. The lines are lowess smoothers, and p values derived from linear regression models adjusted for body size cluster (body mass index and waist circumference), socioeconomic status cluster (mother's age at baseline, mother's age at index birth, race, education, and income), and smoking cluster (smoking during pregnancy (yes/no), smoked, number of years smoked (among ever smokers), amount smoked during pregnancy, current/not current smoking, ever smoking (yes/no), number of cigarettes).
We then considered that higher atherogenic lipids (higher ApoB, total cholesterol, LDL-C, and triglycerides) and blood pressure may explain this early increase in IMT among women with a prior preterm birth. Adjustment for these factors attenuated the preterm vs. term difference in IMT among those with indicated PTB and those with PTB <34 weeks by 30%–40%, suggesting that higher IMT among these groups may result in part from these factors. There was evidence of interaction on the additive scale between the lipid cluster and PTB (p for interaction, 0.07) but not for the interaction of SBP and preterm birth (p for interaction >0.30). Stratified analysis revealed that higher IMT among women with indicated or earlier PTBs was limited to those with LDL >130 mg/dL (p-value for comparisons to term births 0.04 and 0.10, respectively). In contrast, IMT was not higher among those with PTBs vs. term births when LDL was below this threshold (p-values ranging from 0.45 to 0.92; data available upon request).
Among women with a prior preterm birth, only those with deliveries <34 weeks had lower FMD compared to those with term births (difference −1.666, p=0.07), but this difference was not statistically significant. In the adjusted linear models, FMD tended to decrease as gestational age of the index birth decreased, but again this trend did not reach statistical significance (beta 0.17, p=0.06; Fig. 1[B]). PWV was not different among women with preterm vs. term births; nor was there evidence of arterial stiffness as the gestational age of the index pregnancy decreased.
Discussion
In the decade following pregnancy, women with a prior medically indicated preterm birth, as well as those who delivered before 34 weeks gestation, had higher IMT than did women with term births. Cases of preeclampsia and SGA were excluded, and thus medical indications for these PTB included a variety of maternal and fetal conditions that also appear to be related to later-life maternal cardiovascular risk. Higher blood pressure, higher atherogenic lipids, and higher IMT in some women with prior PTB may link this common pregnancy complication to future maternal coronary disease.
Although epidemiologic evidence has associated PTB with excess maternal CVD risk, very few studies of potential mechanisms link these conditions. Our current findings of higher blood pressure, higher lipids, and higher IMT are consistent with postpartum studies of women following pregnancies complicated by preeclampsia.32,33 A third of normotensive PTBs are associated with placental abnormalities commonly found in preeclampsia,34,35 suggesting some shared pathophysiology between these pregnancy complications that may be related to excess maternal CVD risk. In contrast to our current findings, Fraser et al. recently reported that higher blood pressure assessed 18 years after preterm vs. term births in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort in England was explained by hypertension in pregnancy.6 Perhaps different study populations with different predisposing risk for both preterm birth and cardiovascular disease may explain our discordant findings. For example, 30% of women enrolled in our study were African American, a group with excess preterm births, hypertension, and cardiovascular disease. Our findings of impaired IMT and perhaps FMD among women with preterm births delivered before 34 weeks gestation are aligned, however, with other reports that earlier preterm births are associated with particularly high cardiovascular disease risk even after accounting for preeclampsia.3
IMT is a surrogate parameter for risk-factor load as opposed to a tool to detect stable vs. unstable plaques and therefore is well suited to estimate cardiovascular risk among otherwise healthy young adults many years before clinically relevant plaques develop.14 Increasing number of metabolic syndrome components36 and elevated LDL-C13 have been reported to be the dominant factors related to increased carotid-wall thickness among young adults. In our data, women with prior PTBs had higher atherogenic lipid concentrations and higher blood pressure, which may in part explain the higher IMTs we detected among this group. Indeed, higher IMTs among women with indicated PTB or those delivered before 34 weeks was limited to women with borderline high LDL (>130 mg/dL) assessed at the same time as the IMT measurement. Although the blood pressure, lipid, and IMT differences in women with and without PTBs were modest, they are of similar magnitude to preclinical differences that have been associated with the accumulation of CVD risk among young adults.37–38 It is possible that higher lipids and SBP may temporally precede preterm birth, but we were unable to directly study this possibility. Regardless of the temporal nature, however, we treated the lipid cluster and SBP as potentially on the causal pathway linking preterm birth to subclinical vascular disease and therefore accounted for them as intermediaries rather than confounders. Longer follow-up is needed to clarify the temporal nature of the relationship among atherogenic lipids, higher blood pressure, and atherosclerosis in women with prior PTBs. In addition, our results cannot rule out the possibility that the risk of maternal CVD associated with prior preterm delivery may not be conferred completely through classical CVD-risk factors.
Our current study cannot evaluate whether higher IMT preceded or was altered by the PTB, but we propose that a prepregnancy atherogenic phenotype may be related to PTB during the reproductive years, persist or worsen postpartum, and be related to excess CVD later in life. PTB is a common pregnancy complication and may be a relatively simple marker of women at excess cardiometabolic risk who could be identified at a time when lifestyle changes may reverse or delay atherogenesis. Recent guidelines to classify heart disease risk in women included, for the first time, a history of preeclampsia or gestational diabetes as a major risk factor.39 Our data suggest that PTB may also be considered in this group of pregnancy risk factors. Indeed, weight reduction, salt reduction, increased physical activity, smoking cessation, and stress reduction all have been demonstrated to lower blood pressure and atherogenic lipids, as well as to reverse IMT impairments.40–42 Future studies that apply these interventions to women in the decade following a preterm birth are warranted.
IMT, but not PWV, was higher in women with preterm vs. term births. One possibility is that PWV may be less accurately measured compared to IMT, or perhaps these tests detect different pathologic cardiovascular processes. Thicker IMTs are a measure of early arterial response to lipid deposits before a focal plaque may be detected,43 as well as an adaptation to the tensile stress associated with hypertension.44 Thus, IMT is a marker of coronary heart disease development. PWV is related to insulin resistance, blood pressure, and excess stroke risk. Interestingly, in one large observational study, PTB was associated with excess maternal coronary heart disease deaths but not with excess stroke deaths after accounting for infant birth weight.45 FMD was impaired only marginally in women in our study with PTB compared to term controls, suggesting either more variability in this test or that the subtle endothelial dysfunction differences between groups may not yet be robustly detectable in women at a mean age of 38. Reliability studies using similar imaging and reading methodology for endothelium-dependent FMD of the brachial artery have yielded moderate to good intrasonographer reproducibility. These reliability studies include studies within the lab utilized in our study among younger (intraclass correlation coefficient [ICC]=0.72)46,47 and older (ICC=0.7) middle-aged women,48 as well as studies carried out in other labs among diabetic middle-aged adults (CV=29.7%),49 healthy young adult volunteers (ICC=0.6),50 and a subset of the MESA cohort (ICC=0.54).51 Follow-up studies that evaluate progression of vascular impairment according to PTB history are needed to better understand whether our findings of borderline significance result from chance or perhaps are early indications of endothelial-dysfunction risk.
Limitations of our study include modest enrollment of eligible women, which could impair generalizability. The largest hurdle to enrollment was locating current contact information for eligible participants; therefore, our study may reflect associations among a more stable population and thus bias the findings toward the null. Our findings, however, should be replicated in other populations. We evaluated subclinical markers of cardiovascular disease; although these markers have a demonstrated relation to clinical events, additional longitudinal follow-up to study clinical events is needed. In addition, we were unable to determine whether vascular and lipid differences according to prior preterm birth were present before or during pregnancy. We did not have these data; however, future studies should include prepregnancy and during-pregnancy assessments to determine whether there is a common predisposing phenotype to preterm birth and later-life subclinical CVD. Strengths of our study include a relatively large group of racially diverse women with pregnancy data abstracted from hospital birth records and direct assessment of lipids and vascular function 4–12 years after delivery.
Conclusions
Our results demonstrate that in the decade following pregnancy, women with a prior PTB not complicated by preeclampsia have modestly higher blood pressure, atherogenic lipids, and IMT compared to women with term births. Risk was limited to women with non-preeclamptic- indicated preterm births and those with earlier preterm deliveries. Subgroups of women with preterm birth may have excess cardiovascular risk, perhaps warranting screening and lifestyle interventions to prevent future maternal coronary disease.
Acknowledgments
This work was supported by the National Heart, Blood, and Lung Institute at the National Institutes of Health (grant R01HL076532) and the Eunice Kennedy Shriver National Institute of Child Health and Development at the National Institutes of Health (grant K12HD043441).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Irgens H. Reisaeter L. Irgens L. Lie R. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith G. Pell J. Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonamy A-KE. Parikh NI. Cnattingius S. Ludvigsson JF. Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: Clinical perspective. Circulation. 2011;124:2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 4.Catov JM. Wu CS. Olsen J. Sutton-Tyrrell K. Li J. Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–609. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hastie CE. Smith GC. MacKay DF. Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750,350 singleton pregnancies. Int Epidemiol. 2011;40:914–919. doi: 10.1093/ije/dyq270. [DOI] [PubMed] [Google Scholar]
- 6.Fraser A. Nelson SM. Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: Clinical perspective. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catov JM. Bodnar L. Kip K, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:610.e1–7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Catov JM. Ness RB. Wellons MF. Jacobs DR. Roberts JM. Gunderson EP. Prepregnancy lipids related to preterm birth risk: The Coronary Artery Risk Development in Young Adults study. J Clin Endocrinol Metab. 2010;95:3711–3718. doi: 10.1210/jc.2009-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edison RJ. Berg K. Remaley A, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120:723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 10.Magnussen EB. Vatten LJ. Myklestad K. Salvesen KÅ. Romundstad PR. Cardiovascular risk factors prior to conception, the length of pregnancy: Population-based cohort study. Am J Obstet Gynecol. 2011;204:526.e521–526.e528. doi: 10.1016/j.ajog.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Catov JM. Dodge R. Yamal JM. Roberts JM. Piller LB. Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol. 2011;117:225–232. doi: 10.1097/AOG.0b013e3182075626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 13.Li S. Chen W. Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz MW. von Kegler S. Steinmetz H. Markus HS. Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 15.Urbina EM. Srinivasan SR. Tang R. Bond MG. Kieltyka L. Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS. Sorensen KE. Goooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 17.Kuvin JT. Patel AR. Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J American Coll Cardiol. 2001;38:1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 18.Bhuiyan AR. Srinivasan SR. Chen W. Paul TK. Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: The Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. doi: 10.1016/j.atherosclerosis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira I. Boreham CA. Twisk JW, et al. Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: The Northern Ireland Young Hearts Project. J Hypertens. 2007;25:1009–1020. doi: 10.1097/HJH.0b013e3280a94e76. [DOI] [PubMed] [Google Scholar]
- 20.Lebrun CE. van der Schouw YT. Bak AA, et al. Arterial stiffness in postmenopausal women: Determinants of pulse wave velocity. J Hypertens. 2002;20:2165–2172. doi: 10.1097/00004872-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald W. Levy R. Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Sonka M. Liang W. Lauer R. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Trans Med Imaging. 2002;21:1271–1279. doi: 10.1109/TMI.2002.806288. [DOI] [PubMed] [Google Scholar]
- 23.Laurent S. Cockcroft J. Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 24.Allain C. Poon L. Chan C. Richmond W. Fu P. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 25.Bucolo G. David H. Quantitive determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 26.Warnick G. Albers J. A comprehensive evaluation of heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 27.Bingley CA. Gitau R. Lovegrove JA. Impact of Menstrual Cycle Phase on Insulin Sensitivity Measures and Fasting Lipids. Horm Metab Res. 2008;40:901–906. doi: 10.1055/s-0028-1082081. [DOI] [PubMed] [Google Scholar]
- 28.Valdes CT. Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72:642–646. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 29.Sesso HD. Paffenbarger RS. Ha T. Lee IM. Physical activity and cardiovascular disease risk in middle-aged and older women. Am J Epidemiol. 1999;150:408–416. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE. New York: Springer-Verlag; 2001. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. [Google Scholar]
- 31.Shistermam E. Cole S. Platt R. Overadjustment bias and unnecessary ajustment in epidemiologic studies. Epidemiol. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattar N. Ramsay J. Crawford L. Cheyne H. Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 33.Wilson BJ. Watson MS. Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias F. Rodriquez L. Rayne SC. Kraus FT. Maternal placental vasculopathy and infection: Two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 35.Germain AM. Carvajal J. Sanchez M. Valenzuela GJ. Tsunekawa H. Chuaqui B. Preterm labor: Placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 36.Tzou WS. Douglas PS. Srinivasan SR, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: The Bogalusa Heart Study. J Am Coll Cardiol. 2005;46:457–463. doi: 10.1016/j.jacc.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Kestilä P. Magnussen CG. Viikari JSA, et al. Socioeconomic status, cardiovascular risk factors, and subclinical atherosclerosis in young adults: The Cardiovascular Risk in Young Finns Study. Arterioscler, Thromb, and Vasc Biol. 2012;32:815–821. doi: 10.1161/ATVBAHA.111.241182. [DOI] [PubMed] [Google Scholar]
- 38.Pletcher MJ. Bibbins-Domingo K. Liu K, et al. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: The CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med. 2010;153:137–146. doi: 10.1059/0003-4819-153-3-201008030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosca L. Benjamin EJ. Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildman RP. Schott LL. Brockwell S. Kuller LH. Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardio. 2004;44:579–585. doi: 10.1016/j.jacc.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 41.Bigornia SJ. Mott MM. Hess DT, et al. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring) 2010;18:754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang CQ. Xu L. Lam TH. Lin JM. Cheng KK. Thomas GN. Smoking cessation and carotid atherosclerosis: The Guangzhou Biobank Cohort Study—CVD. J Epidemiol Comm Health. 2010;64:1004–1009. doi: 10.1136/jech.2009.092718. [DOI] [PubMed] [Google Scholar]
- 43.Kolodgie FD. Burke AP. Nakazawa G. Virmani R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler Thromb Vasc Biol. 2007;27:986–989. doi: 10.1161/ATVBAHA.0000258865.44774.41. [DOI] [PubMed] [Google Scholar]
- 44.Najjar SS. Scuteri A. Lakatta EG. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 45.Smith DG. Sterne J. Tynelius P. Lawlor DA. Rasmussen F. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiology. 2005;16:563–569. doi: 10.1097/01.ede.0000164790.96316.c0. [DOI] [PubMed] [Google Scholar]
- 46.Harris KF. Matthews KA. Sutton-Tyrrell K. Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–409. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 47.Thurston RC. Sutton-Tyrrell K. Everson-Rose SA. Hess R. Matthews KA. Hot flashes and subclinical cardiovascular disease. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loehr LR. Espeland MA. Sutton-Tyrrell K. Burke GL. Crouse Iii JR. Herrington DM. Racial differences in endothelial function in postmenopausal women. Am Heart J. 2004;148:606–611. doi: 10.1016/j.ahj.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Donald AE. Halcox JP. Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Peretz A. Leotta D. Sullivan J, et al. Flow mediated dilation of the brachial artery: An investigation of methods requiring further standardization. BMC Cardiovasc Disord. 2007;7:11. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeboah J. Folsom AR. Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]