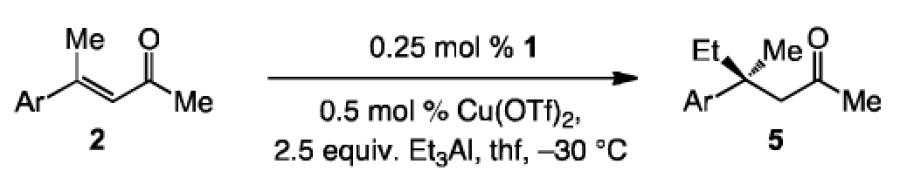
Table 2.
NHC-Cu-catalyzed ECA of aryl-substituted enones with Et3AI.[a]
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ar; Substrate | Product | t[h] | Conv. [%];[b]
Yield [%][c] |
e.r.[d] |
| 1 | Ph; 2b | 5a | 0.5 | >98; 93 | 98:2 |
| 2 | 2-thienyl; 2c | 5b | 1.0 | >98; 86 | 98.5:1.5 |
| 3 | pF3CC6H4; 2e | 5c | 1.0 | >98; 89 | 99:1 |
| 4 | pMeOC6H4; 2f | 5d | 1.0 | >98; 92 | 99:1 |
| 5 | 2-naphthyl; 2h | 5e | 2.5 | 97; 94 | 97.5:2.5 |
| 6 | oBrC6H4; 2i | 5f | 12 | 98; 87 | >99:1 |
| 7 | mFC6H4; 2j | 5g | 3.0 | >98; 90 | 96.5:3.5 |
Reactions were performed under N2 atmosphere.
Determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures.
Yield of isolated and purified products.
Determined by GC analysis (entries 1-2) or HPLC analysis (±2%); see the Supporting Information for details.
