Figure 4. Targeted Knockdown of Vps45 with the Use of Morpholino Oligonucleotides (MOs) That Block Splicing and Translation in Zebrafish Embryos.
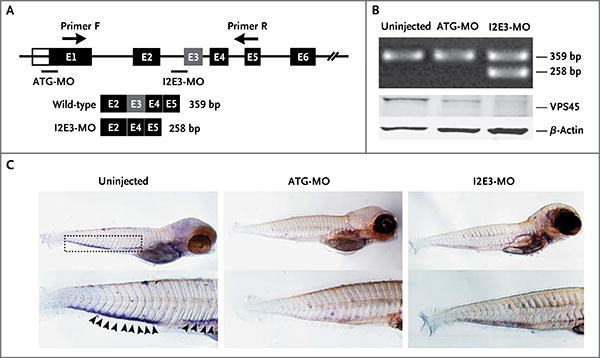
Panel A is a diagram of morpholino design showing the zebrafish gene vps45 and morpholino antisense strategies to block either the translation of the zebrafish vps45 messenger RNA (ATG-MO) or the splice acceptor site of exon 3 (I2E3-MO). Primers F and R validate exon skipping, including the expected size of amplicons that result from loss of exon 3. Below the diagram is a schematic depiction of the spliced transcript in the I2E3-MO–injected embryos (258 bp) as compared with the uninjected and ATG-MO–injected embryos (359 bp). In Panel B, the top immunoblot shows the results of reverse-transcriptase–polymerase-chain-reaction analysis of vps45 transcript from uninjected and morpholino-injected embryos 5 days after fertilization. The bottom immunoblot shows the results of Western blot analysis of whole-protein lysates from uninjected and morpholino-injected embryos 5 days after fertilization. Lysates from embryos injected with ATG-MO and from those injected with I2E3-MO show marked reduction of vps45 when normalized with β-actin, which was used as a protein-loading control. In Panel C, in situ hybridization of embryos 5 days after fertilization supports functional knockdown of vps45. Shown are representative images of uninjected zebrafish embryos (left), embryos injected with ATG-MO (middle), and embryos injected with I2E3-MO (right). Results of whole-mount in situ hybridization with the use of a digoxigenin-labeled RNA probe against zebrafish myeloperoxidase are shown. The myeloperoxidase detects neutrophils in the caudal hematopoietic tissue (rectangle). In uninjected embryos, the myeloperoxidase signals are readily seen (arrowheads). However, in ATG-MO–injected and I2E3-MO–injected embryos, there is a marked reduction in myeloperoxidase staining, suggestive of a decreased number of mature neutrophils.
