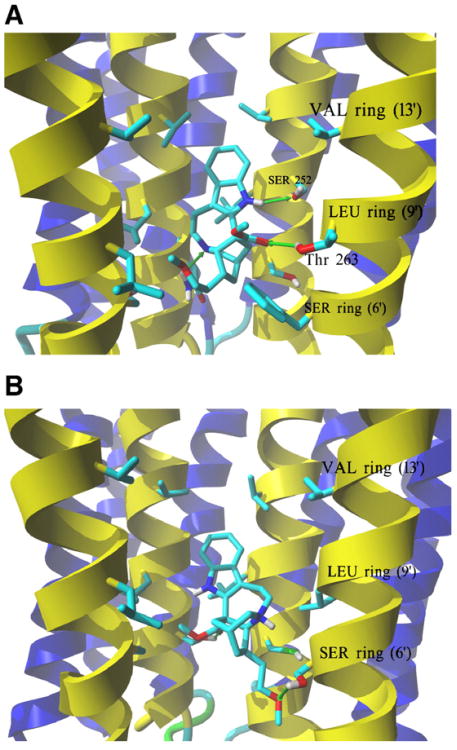
Fig. 7.
Complexes formed between 18-MC and AChR ion channel models obtained by molecular docking. (A) Side view of the lowest energy complex formed between neutral 18-MC and the human muscle AChR ion channel. Receptor subunits are shown in the secondary structure mode (M2 helices, yellow; other transmembrane helices, blue) with residues forming the serine (SER) (position 6′), leucine (LEU) (positions 9′), and valine (VAL) (position 13′) rings, shown explicitly in stick mode. Green arrows indicate hydrogen bonds formed between the pyrrole amino group and the α1-Ser252 residue (position 10′), another between the ligand ionizable amino group and γ-Asn257 at the SER ring, and a third one between the carbonyl of the ester group and β1-Thr263 (position 10′). A similar interaction was obtained for protonated 18-MC. (B) Side view of the lowest energy complex formed between protonated 18-MC and the Torpedo AChR ion channel. The subunit γ was hidden for clarity, and the order of the remaining subunits is (from the left): α1, β1,δ, α1. The ether and ester groups from 18-MC form hydrogen bonds with two Ser residues, each one from the respective α1 and β1 subunits, forming the SER ring. Another strong hydrogen bond interaction is observed between the charged amino group and the same α1-Ser residue. 18-MC interacts with the LEU and VAL rings by additional weaker van der Waals contacts. A similar interaction was obtained for neutral 18-MC.