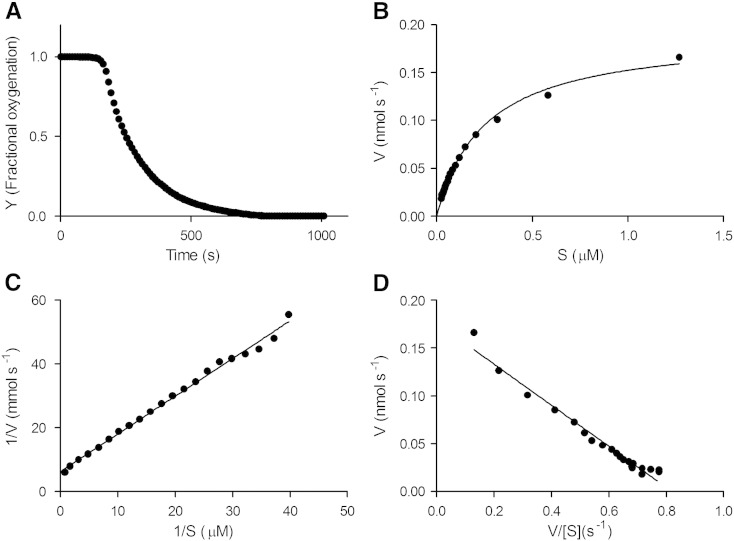
Fig. 6.
Determination of oxygen affinity of cytochrome bd-II in membranes by the deoxygenation of oxyleghaemoglobin. (A) Deoxygenation of oxyleghaemoglobin during respiration of membranes containing cytochrome bd-II as the only oxidase (reaction stimulated with NADH, 3 mM final concentration). (B) Oxygen consumption rates (V) and oxygen concentrations (S) were derived from the Appleby and Bergersen [77] equations. (C) Lineweaver–Burk plot and (D) Eadie-Hofstee plot. The affinity of cytochrome bd-II for oxygen (Km(O2)) was determined to be 0.24 μM (SD 0.019), with a Vmax value of ~ 0.2 nmol s− 1 mg protein− 1 (SD 0.02).