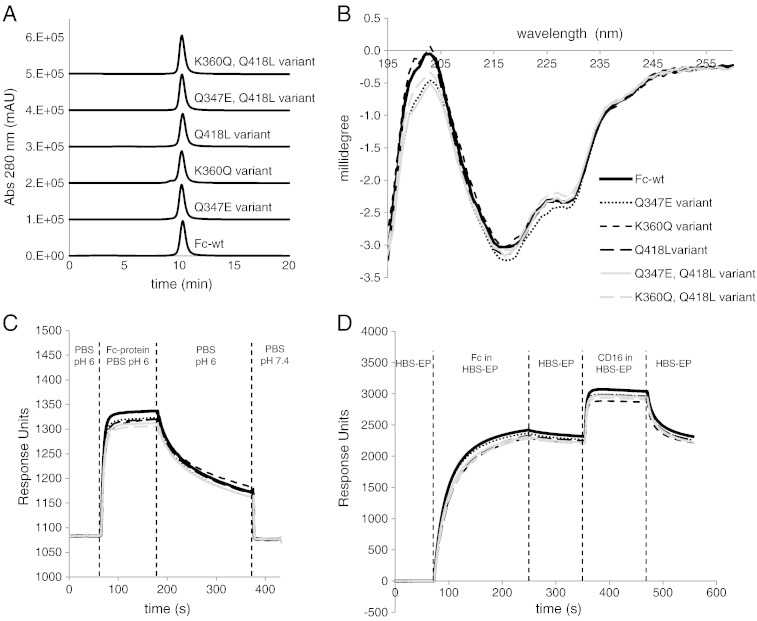
Fig. 6.
Characterization of stabilized Fc variants compared to Fc-wt. (A) SEC-HPLC analysis of Fc-wt and selected Fc variants. One representative experiment out of two is shown. (B) Comparison of electronic circular dichroism (ECD) far-UV spectra of Fc-wt and stabilized Fc variants in the range of 260–195 nm. Average spectra from three experiments are shown. (C) Qualitative SPR analysis of FcRn binding of Fc-wt and stabilized Fc variants. An FcRn coated SPR-chip was incubated with PBS pH 6.0 (baseline), followed by injection of Fc-samples in PBS pH 6.0 (association), PBS pH 6.0 only and PBS pH 7.4 only for analysis of the pH-dependent dissociation. (D) Qualitative evaluation of Protein A and CD16a binding properties of Fc samples. Protein A was immobilized onto an SPR-chip. After measuring the baseline with HBS-EP buffer (pH 7.4), association with Protein A was analyzed by injection of Fc samples (HBS-EP buffer), followed by analysis of the dissociation of the Fc proteins from Protein A by injection of HBS-EP buffer only. Subsequently, association with CD16a was analyzed by incubation with the respective ligand, followed by dissociation analysis by injecting HBS-EP buffer only. In C and D average values from two experiments are shown.