Figure 6.
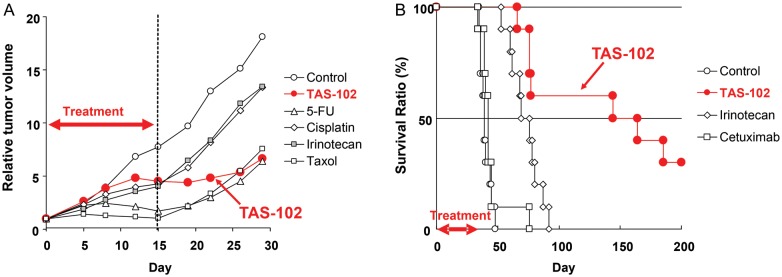
Antitumor effects of TAS-102 in preclinical xenograft models. TAS-102 treatment demonstrated the sustained tumor growth inhibition and survival benefit in preclinical studies. (A) KM20C xenograft model in mice; drugs were administered to mice subcutaneously implanted with a human colon cancer line, KM20C. Dosing schedules were as follows: TAS-102 (150 mg/kg/day, p.o., Days 1–14), 5-FU (15 mg/kg/day, c.i., Days 1–15), cisplatin (7 mg/kg/day, i.v., Days 1,8), irinotecan (40 mg/kg/day, i.v., Days 1 and 8), taxol (30 mg/kg/day, i.v., Days 1 and 8). (B) KM20C survival model in mice; Drugs were administered to mice intraperitoneally implanted with KM20C. Dosing schedules were as follows: TAS-102 (200 mg/kg/day, p.o., five consecutive days followed by 2 days rest a week for 6 weeks), irinotecan (100 mg/kg/day, i.v., once a week for 6 weeks) and cetuximab (40 mg/kg/day, i.p., twice a week for 6 weeks).
